Highly electron-poor Buchwald-type ligand: application for Pd-catalysed direct arylation of thiophene derivatives and theoretical consideration of the secondary Pd0–arene interaction†
Abstract
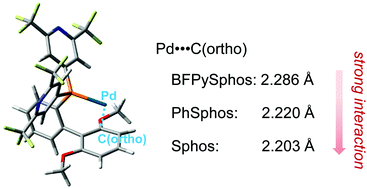
Highly electron-poor SPhos ligands bearing either 2,6-bis(trifluoromethyl)-4-pyridyl (BFPy) or 3,5-(CF3)2C6H3 groups were synthesised. The former ligand highly accelerated the Pd-catalysed direct arylation of 2-propylthiophene, 2-methylthiophene or benzo[b]thiophene with only 1 mol% of the catalyst. This high catalytic activity can be attributed to a combination of electronic properties and the secondary Pd–arene interaction of BFPySPhos. The secondary interactions of SPhos, PhSPhos and BFPySPhos were optimised at the oniom(mp2/lanl2dz : b3lyp/lanl2dz) level and were further evaluated using the NBO method by DFT at the M06-2X/6-31G(d) level with LanL2DZ + ECP. The deletion energy analysis showed that the transfer of electrons from Pd to aromatic ring is the dominating factor for the secondary Pd–arene interaction of SPhos–Pd0 complexes. Although an electron-poor BFPySPhos does not particularly favour this type of interaction, this interaction is still substantial enough to sufficiently stabilise the BFPySPhos–Pd complex.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...