Synthesis of new asymmetric substituted boron amidines – reactions with CO and transfer hydrogenations of phenylacetylene†
Abstract
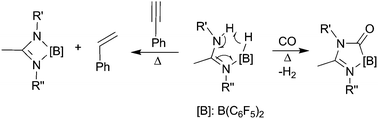
The syntheses of the new asymmetric substituted boron amidines [N′-(2,6-diisopropylphenyl)-N-(pentafluorophenyl)acetimidamide]bis(pentafluorophenyl)borate (2a) and [N′-(2,6-diisopropylphenyl)-N-(4-cyanophenyl)acetimidamide]bis(pentafluorophenyl)borate (2b) were achieved by reaction of one equivalent of HB(C6F5)2 and the respective amidines 1a and 1b. These adducts, bearing electron withdrawing groups, showed thermally induced H2 elimination forming the four-membered cyclic diazaborate derivatives 3a and 3b. These new species were characterized by spectroscopic methods. X-ray diffraction studies have been carried out on 2a, 2b and 3a. To prevent undesired reactions at the nitrile group, one equivalent of B(C6F5)3 was added to 2b yielding the 2b–B(C6F5)3 nitrile adduct 4. Compound 4 underwent thermally induced dehydrogenation to give the four-membered cyclic diazaborate derivative 5. CO was inserted into the ring systems of 2a and 2b forming the five-membered diazaborolone derivatives 6a and 6b. Phenylacetylene reacted stoichiometrically with the asymmetric substituted boron amidines 2a, 2b and 4 to give styrene by double H transfer.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...