Synthesis of Ir(iii) complexes with TpMe2 and acac ligands and their reactivity with electrophiles†
Abstract
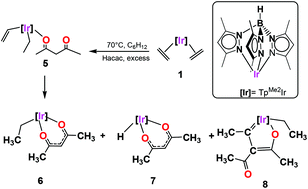
The reaction of the bis(ethylene) complex [TpMe2Ir(C2H4)2] (1) (TpMe2 = hydrotris(3,5-dimethylpyrazolyl)borate) with an excess of 2,4-pentanedione (acetylacetone, Hacac) at 70 °C produced a mixture of the Ir(III) complex [TpMe2Ir(acac)(C2H5)] (6) as a major product (67% yield) and two other side complexes [TpMe2Ir(acac)(H)] (7) and [TpMe2Ir(C9H14O2)] (8) in 20 and 13% yields, respectively. According to the proposed reaction mechanism and DFT calculations, complexes 6 and 7 are generated from an 18e− intermediate [TpMe2Ir(C2H4)(acac)(C2H3)] (5) which undergoes either hydrogen insertion or β-hydride elimination followed by the subsequent loss of a molecule of ethylene. The lowest yielding complex 8 which features a 2-iridafuran is presumably generated from an unusual thermal coupling between one vinylic and one acac moiety. The availability of the acidic α-proton of the acac ligand was tested by the treatment of complex 6 with Br2 and Cu(NO3)2 rendering the substitution complexes [Tp3-Br,Me2Ir(3-Br-acac)Br] (9) and [TpMe2Ir(3-NO2-acac)(C2H5)] (10) in good yields. The series of heteroleptic iridium(III) compounds display air and moisture stability and have been characterized by NMR, IR, and elemental analyses, and, in the case of 6, 8 and 9, by single-crystal X-ray diffraction analyses.

 Please wait while we load your content...
Please wait while we load your content...