Open Access Article
Open Access ArticleRu–Ag and Ru–Au dicarbene complexes from an abnormal carbene ruthenium system†
Mario J.
Bitzer
a,
Alexander
Pöthig
a,
Christian
Jandl
a,
Fritz E.
Kühn
*a and
Walter
Baratta
*b
aInorganic Chemistry/Molecular Catalysis, Department of Chemistry & Catalysis Research Center, Technische Universität München (TUM), Lichtenbergstr. 4, D-85747 Garching bei München, Germany. E-mail: fritz.kuehn@ch.tum.de
bDipartimento di Chimica, Fisica e Ambiente, Università di Udine, Via Cotonificio 108, I-33100 Udine, Italy. E-mail: walter.baratta@uniud.it
First published on 1st June 2015
Abstract
Reaction of [Ru(OAc)2(PPh3)2] with a P-functionalized imidazolium bromide easily affords a cationic abnormal carbene Ru system. Metalation with Ag2O yields a Ru–Ag complex containing an anionic dicarbene ligand, while subsequent transmetalation with Au(tht)Cl leads to the corresponding Ru–Au system. The bimetallic complexes were characterized by single crystal X-ray diffraction and are the first examples of complexes bearing anionic dicarbene ligands connecting two different d-block elements.
N-heterocyclic carbenes (NHCs) have been widely employed as practical ligands in organometallic chemistry and in catalysis during the last two decades.1–12 Imidazol-2-ylidene NHC ligands exhibit unique features with respect to stability, donor strength and steric requirements, leading to several coordination modes (Fig. 1).
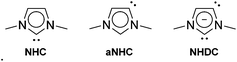 | ||
| Fig. 1 Possible coordination sites of unsaturated imidazol-2-ylidene ligands: (NHC) normal carbene, (aNHC) abnormal carbene, (NHDC) anionic dicarbene. | ||
While the normal carbene coordination (NHC) still remains predominant in transition metal complexes,1–12 the abnormal coordination mode (aNHC) was first reported by Crabtree in 2001,13 and several derivatives were isolated subsequently.14,15 For Ru only a few aNHC-species have been described,16–21 and some display high catalytic activity in transfer hydrogenation.17 Compared to NHCs, the aNHC ligands show stronger σ-donating properties and therefore are complementary ligands of widespread relevance for catalysis. The third type of coordination was first described by Arnold in 2006 for lanthanide–potassium complexes, in which anionic dicarbenes (NHDC) have both a normal and an abnormal carbene center,22 and other examples have been discovered based on main-group elements.23–26 NHDC complexes containing one d-block and one main group element have been reported very recently by Goicoechea,27–29 Stephan30 and Tamm.31 In addition, examples with two d-block elements are those based on Pd–Pd, Zn–Zn, Ru–Ru, Ir–Ir and Au–Au systems.21,32–37 By contrast, no hetero-bimetallic NHDC complexes with two different d-block elements have been reported to date. Interestingly, hetero-bimetallic complexes, based on the related 1,2,4-triazolyl-3,5-diylidene carbenes, have been applied in tandem catalysis, and show superior performance compared to a combination of monometallic species due to cooperative effects.38,39
Here we report the isolation of the first example of a NHDC complex with two different d-block elements, starting from a cationic aNHC Ru complex and Ag2O. Transmetalation of the Ru–Ag derivative results in the formation of a Ru–Au complex, enlarging the synthetic scope for NHDC complexes.
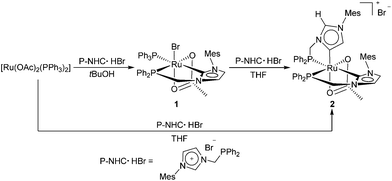
The cationic abnormal NHC ruthenium complex 2 can be easily obtained starting from [Ru(OAc)2(PPh3)2] and a functionalized imidazolium bromide (P-NHC·HBr),40 containing one CH2 bridged phosphine arm, via the normal NHC compound 1 (Scheme 1).
Treatment of [Ru(OAc)2(PPh3)2] with one equivalent of P-NHC·HBr and NaOAc in tBuOH at reflux (3 h) affords the NHC complex 1 in 85% yield. A cis RuP2 arrangement has been assigned on the basis of the small 2JPP = 23.6 Hz. The 13C{1H} NMR carbene shows signals at δ = 190.3 ppm, as a doublet of doublets with 2JCP = 109.6 and 11.6 Hz, for trans and cis P atoms, respectively, thus establishing the geometry of 1.41 It is worth pointing out that the reaction of [Ru(OAc)2(PPh3)2] with the analogous P-functionalized imidazolium bromide with a CH2CH2 bridge led to an abnormal NHC Ru complex.17 Reaction of 1 with one equivalent of P-NHC·HBr in the presence of NaOAc in THF at reflux affords 2 (80% yield) by substitution of PPh3 and bromide with the chelating aNHC ligand. The two P doublets of 2 at δ = 79.5 and 62.9 ppm (2JPP = 23.9 Hz) are consistent with the presence of a cis RuP2 geometry. The 13C{1H} NMR spectrum shows a doublet of doublets at δ = 191.6 ppm with 2JCP = 102.5 and 11.4 Hz, for a normal carbene with trans and cis P atoms, and a doublet of doublets at high field δ = 163.0 ppm (2JCP = 18.4 and 8.5 Hz) for an abnormal carbene with two cis P atoms. In the 1H NMR spectrum, the signal of the NCHN proton of the abnormal carbene is exceptionally downfield shifted at δ = 9.75 ppm, close to that of the ligand precursor P-NHC·HBr (10.33 ppm), suggesting a relatively acidic proton.42–44 Complex 2 can also be prepared directly from [Ru(OAc)2(PPh3)2] and 2 equiv. of P-NHC·HBr in THF in the presence of NaOAc (88% yield). Notably, 2 is a rare example of a Ru-NHC complex bearing both a normal and an abnormal carbene ligand,16 which may form on account of steric factors involving the bulky mesityl group.
Preliminary results show that 2 (0.1 mol%) displays high catalytic activity in the transfer hydrogenation (TH) of ketones. In the presence of NaOiPr (2 mol%) in 2-propanol at reflux,40 acetophenone, benzophenone and cyclohexanone are reduced to the corresponding alcohols with 97, 90 and 99% conversion in 20, 80 and 5 min respectively, achieving a TOF up to 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. These results indicate that 2 is among the most active Ru carbene TH catalysts,17,45–48 even without an amine N–H function which is usually introduced to obtain high activity (bifunctional catalysis).49,50
000 h−1. These results indicate that 2 is among the most active Ru carbene TH catalysts,17,45–48 even without an amine N–H function which is usually introduced to obtain high activity (bifunctional catalysis).49,50
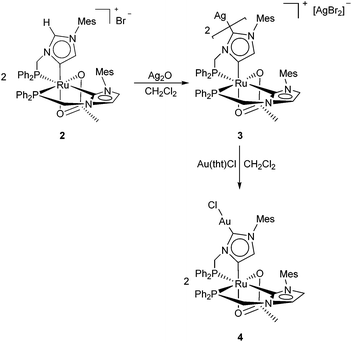
Since the cationic aNHC complex 2, displaying an NCHN proton, can be considered as an “imidazolium salt”, we investigated the reaction of 2 with Ag2O in order to achieve deprotonation and coordination of an Ag atom at the C2 carbon of the abnormal carbene ligand (Scheme 2).
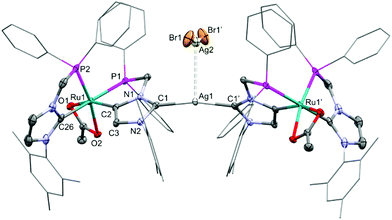
To our delight, the hetero-bimetallic anionic dicarbene 3 was cleanly obtained in 92% yield by stirring a suspension of 2 (2 equiv.) and Ag2O in CH2Cl2 at RT for 7 days, and was characterized by NMR and single crystal X-ray diffraction (Fig. 2).40 The crystal structure of 3 shows that two Ru units are almost linearly (C1–Ag1–C1′ = 167.8(3)°) linked by the Ag1 atom via the anionic dicarbenes. The Ru atoms are coordinated in a pseudo-octahedral geometry, with the same set of ligands as 2. The Ag1–C1 bond length (2.112(5) Å) is slightly longer compared to other Ag carbenes,51,52 whereas the Ru1–C2 distance (2.022(5) Å) is similar to that of the related abnormal Ru-aNHC complex17 and shorter than that of the carbene Ru1–C26 bond (2.089(5) Å). An argentophilic interaction between the [AgBr2]− anion and Ag1 is indicated by an Ag1–Ag2 distance of 3.1211(8) Å.53 Thus, this is the first example of an NHDC complex containing two different d-block elements.
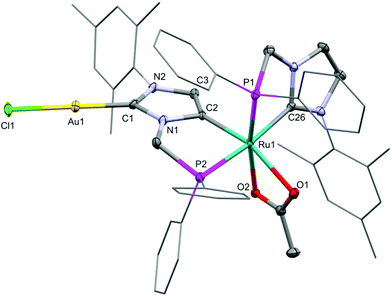
Silver carbene complexes have frequently been employed as transmetalation reagents for the preparation of a large number of NHC complexes.12,54 Treatment of 3 with 2 equiv. of the gold precursor Au(tht)Cl (tht = tetrahydrothiophene) in CH2Cl2 at RT (2 d) afforded the gold NHDC derivative 4 in 93% yield (Scheme 2). Upon transmetalation, the Ru–Ag–Ru unit of 3 is split up into two Ru–Au fragments. The gold atom is linearly coordinated by a chloride and the C1-atom of the anionic dicarbene, exhibiting an Au1–C1 distance of 1.984(4) Å and a C1–Au1–Cl1 angle of 178.74(12)°. Almost identical to 3, the Ru1–C2 distance is 2.018(4) Å (Fig. 3).
The different structures observed for 3 and 4 in the solid state may be associated with subtle effects involving the competition between the neutral [MX(Ru-NHDC)] versus the ionic [M(Ru-NHDC)2][MX2] (M = Ag, Au; X = Cl, Br) form.51 The overall NMR data of 3 and 4 in solution are very similar. However, while for 4 the 13C{1H} NMR signal of the C2 carbon bound to Au is at δ = 167.3 ppm with a 3JCP = of 12.7 Hz, no signal was observed for the C2 of 3 even at −90 °C, possibly due to a dynamic process involving the [AgX2]− counter ion, a behavior which was reported for other Ag-NHC complexes bearing silver halide anions.52,55 It is worth pointing out that the syntheses of 3 and 4 occur under mild reaction conditions without modifying the coordination environment of the cationic aNHC Ru complex 2.
Conclusions
In conclusion, we have reported the first complexes containing an anionic dicarbene ligand connected to two different d-block elements, namely a Ru–Ag and a Ru–Au system. The synthetic pathway entails the use of a cationic abnormal carbene Ru complex, which cleanly undergoes metalation and transmetalation reactions. This Ru derivative was also found to be catalytically active in ketone transfer hydrogenation. Further studies aiming to extend this protocol to the preparation of hetero-bimetallic complexes containing anionic dicarbene ligands via cationic aNHC complexes, and examinations of the catalytic utilization of such compounds are currently underway.We thank the Erasmus STA program and the TUM Graduate School for financial support.
Notes and references
- K. Riener, S. Haslinger, A. Raba, M. P. Högerl, M. Cokoja, W. A. Herrmann and F. E. Kühn, Chem. Rev., 2014, 114, 5215–5272 CrossRef CAS PubMed.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed.
- D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC.
- Y. Wang and G. H. Robinson, Dalton Trans., 2012, 41, 337–345 RSC.
- T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef PubMed.
- J. W. Herndon, Coord. Chem. Rev., 2010, 254, 103–194 CrossRef CAS PubMed.
- G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2009, 110, 1746–1787 CrossRef PubMed.
- S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed.
- F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122–3172 CrossRef CAS PubMed.
- D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed.
- V. Cesar, S. Bellemin-Laponnaz and L. H. Gade, Chem. Soc. Rev., 2004, 33, 619–636 RSC.
- W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1309 CrossRef CAS.
- S. Grundemann, A. Kovacevic, M. Albrecht, J. W. Faller and R. H. Crabtree, Chem. Commun., 2001, 2274–2275 RSC.
- R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755–766 CrossRef CAS PubMed.
- P. L. Arnold and S. Pearson, Coord. Chem. Rev., 2007, 251, 596–609 CrossRef CAS PubMed.
- G. A. Filonenko, E. Cosimi, L. Lefort, M. P. Conley, C. Copéret, M. Lutz, E. J. M. Hensen and E. A. Pidko, ACS Catal., 2014, 4, 2667–2671 CrossRef CAS.
- J. Witt, A. Pöthig, F. E. Kühn and W. Baratta, Organometallics, 2013, 32, 4042–4045 CrossRef CAS.
- S. Saha, T. Ghatak, B. Saha, H. Doucet and J. K. Bera, Organometallics, 2012, 31, 5500–5505 CrossRef CAS.
- L. Benhamou, J. Wolf, V. Cesar, A. Labande, R. Poli, N. Lugan and G. Lavigne, Organometallics, 2009, 28, 6981–6993 CrossRef CAS.
- A. Prades, M. Viciano, M. Sanaú and E. Peris, Organometallics, 2008, 27, 4254–4259 CrossRef CAS.
- C. E. Ellul, M. F. Mahon, O. Saker and M. K. Whittlesey, Angew. Chem., Int. Ed., 2007, 46, 6343–6345 CrossRef CAS PubMed.
- P. L. Arnold and S. T. Liddle, Organometallics, 2006, 25, 1485–1491 CrossRef CAS.
- A. El-Hellani and V. Lavallo, Angew. Chem., Int. Ed., 2014, 53, 4489–4493 CrossRef CAS PubMed.
- Y. Wang, M. Y. Abraham, R. J. Gilliard, P. Wei, J. C. Smith and G. H. Robinson, Organometallics, 2012, 31, 791–793 CrossRef CAS.
- Y. Wang, Y. Xie, M. Y. Abraham, P. Wei, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, Organometallics, 2011, 30, 1303–1306 CrossRef CAS.
- Y. Wang, Y. Xie, M. Y. Abraham, P. Wei, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2010, 132, 14370–14372 CrossRef CAS PubMed.
- R. A. Musgrave, R. S. P. Turbervill, M. Irwin, R. Herchel and J. M. Goicoechea, Dalton Trans., 2014, 43, 4335–4344 RSC.
- J. B. Waters, R. S. P. Turbervill and J. M. Goicoechea, Organometallics, 2013, 32, 5190–5200 CrossRef CAS.
- R. A. Musgrave, R. S. P. Turbervill, M. Irwin and J. M. Goicoechea, Angew. Chem., Int. Ed., 2012, 51, 10832–10835 CrossRef CAS PubMed.
- C. Pranckevicius and D. W. Stephan, Chem. – Eur. J., 2014, 20, 6597–6602 CrossRef CAS PubMed.
- S. Kronig, E. Theuergarten, C. G. Daniliuc, P. G. Jones and M. Tamm, Angew. Chem., Int. Ed., 2012, 51, 3240–3244 CrossRef CAS PubMed.
- D. R. Armstrong, S. E. Baillie, V. L. Blair, N. G. Chabloz, J. Diez, J. Garcia-Alvarez, A. R. Kennedy, S. D. Robertson and E. Hevia, Chem. Sci., 2013, 4, 4259–4266 RSC.
- Y. Wang, Y. Xie, M. Y. Abraham, R. J. Gilliard, P. Wei, C. F. Campana, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, Angew. Chem., Int. Ed., 2012, 51, 10173–10176 CrossRef CAS PubMed.
- A. Krüger, E. Kluser, H. Müller-Bunz, A. Neels and M. Albrecht, Eur. J. Inorg. Chem., 2012, 1394–1402 CrossRef PubMed.
- U. J. Scheele, S. Dechert and F. Meyer, Chem. – Eur. J., 2008, 14, 5112–5115 CrossRef CAS PubMed.
- M. R. Crittall, C. E. Ellul, M. F. Mahon, O. Saker and M. K. Whittlesey, Dalton Trans., 2008, 4209–4211 RSC.
- A. A. Danopoulos, D. Pugh and J. A. Wright, Angew. Chem., Int. Ed., 2008, 47, 9765–9767 CrossRef PubMed.
- J. A. Mata, F. E. Hahn and E. Peris, Chem. Sci., 2014, 5, 1723–1732 RSC.
- S. Sabater, J. A. Mata and E. Peris, Organometallics, 2012, 31, 6450–6456 CrossRef CAS.
- The syntheses and characterization of P-NHC·HBr and the complexes 1–4, as well as the catalytic results, are reported in the ESI.† CCDC 1036993 (compound 3) and CCDC 1036994 (compound 4) contain the supplementary crystallographic data for this paper.
- The 31P{1H} NMR spectrum of 1 shows the presence of two closely related species in about 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, also confirmed by 1H and 13C{1H} NMR measurements, consistent with the presence of two conformers involving the P-NHC five-membered ring. While the 31P{1H} NMR spectrum shows a Δδ < 0.2 ppm for the two species, in the 13C{1H} NMR spectrum the signal which shows the larger difference in chemical shift (Δδ = 0.9 ppm) is for the bridged CH2 group. In addition, a hindered rotation of the Mes group is observed even at 60 °C by 1H NMR. See ESI† for details.
2 molar ratio, also confirmed by 1H and 13C{1H} NMR measurements, consistent with the presence of two conformers involving the P-NHC five-membered ring. While the 31P{1H} NMR spectrum shows a Δδ < 0.2 ppm for the two species, in the 13C{1H} NMR spectrum the signal which shows the larger difference in chemical shift (Δδ = 0.9 ppm) is for the bridged CH2 group. In addition, a hindered rotation of the Mes group is observed even at 60 °C by 1H NMR. See ESI† for details. - T. Guo, S. Dechert and F. Meyer, Organometallics, 2014, 33, 5145–5155 CrossRef CAS.
- K. V. Tan, J. L. Dutton, B. W. Skelton, D. J. D. Wilson and P. J. Barnard, Organometallics, 2013, 32, 1913–1923 CrossRef CAS.
- S. Gründemann, A. Kovacevic, M. Albrecht, J. W. Faller and R. H. Crabtree, J. Am. Chem. Soc., 2002, 124, 10473–10481 CrossRef PubMed.
- A. R. Naziruddin, Z.-J. Huang, W.-C. Lai, W.-J. Lin and W.-S. Hwang, Dalton Trans., 2013, 42, 13161–13171 RSC.
- Y. Cheng, X.-Y. Lu, H.-J. Xu, Y.-Z. Li, X.-T. Chen and Z.-L. Xue, Inorg. Chim. Acta, 2010, 363, 430–437 CrossRef CAS PubMed.
- W. Baratta, J. Schütz, E. Herdtweck, W. A. Herrmann and P. Rigo, J. Organomet. Chem., 2005, 690, 5570–5575 CrossRef CAS PubMed.
- M. Poyatos, J. A. Mata, E. Falomir, R. H. Crabtree and E. Peris, Organometallics, 2003, 22, 1110–1114 CrossRef CAS.
- W. Baratta, S. Baldino, M. J. Calhorda, P. J. Costa, G. Esposito, E. Herdtweck, S. Magnolia, C. Mealli, A. Messaoudi, S. A. Mason and L. F. Veiros, Chem. – Eur. J., 2014, 20, 13603–13617 CrossRef CAS PubMed.
- M. Yamakawa, H. Ito and R. Noyori, J. Am. Chem. Soc., 2000, 122, 1466–1478 CrossRef CAS.
- C. Topf, C. Hirtenlehner, M. Zabel, M. List, M. Fleck and U. Monkowius, Organometallics, 2011, 30, 2755–2764 CrossRef CAS.
- H. M. J. Wang and I. J. B. Lin, Organometallics, 1998, 17, 972–975 CrossRef CAS.
- H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS PubMed.
- J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978–4008 CrossRef CAS PubMed.
- M. V. Baker, D. H. Brown, R. A. Haque, B. W. Skelton and A. H. White, Dalton Trans., 2004, 3756–3764 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR data, catalytic results and crystallographic data. CCDC 1036993–1036994. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt01914a |
| This journal is © The Royal Society of Chemistry 2015 |