 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Methylresorcinarene: a reaction vessel to control the coordination geometry of copper(II) in pyridine N-oxide copper(II) complexes†
Ngong Kodiah
Beyeh
* and
Rakesh
Puttreddy
University of Jyvaskyla, Department of Chemistry, P. O. Box 35, 40014 University of Jyvaskyla, Jyvaskyla, Finland. E-mail: ngong.k.beyeh@jyu.fi; Fax: +358-14-2602501; Tel: +358-40-8053692
First published on 22nd April 2015
Abstract
Pyridine and 2-picolinic acid N-oxides form 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand
1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal (L
metal (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M) discrete L2M2 and polymeric complexes with CuCl2 and Cu(NO3)2, respectively, with copper(II) salts. The N-oxides also form 1
M) discrete L2M2 and polymeric complexes with CuCl2 and Cu(NO3)2, respectively, with copper(II) salts. The N-oxides also form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes with methylresorcinarene. In combination, the three components form a unique 2
1 host–guest complexes with methylresorcinarene. In combination, the three components form a unique 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–ligand–metal complex. The methylresorcinarene acts as a reaction vessel/protecting group to control the coordination of copper(II) from cis-see-saw to trans-square planar, and from octahedral to square planar coordination geometry. These processes were studied in solution and in the solid state via1H NMR spectroscopy and single crystal X-ray diffraction.
1 host–ligand–metal complex. The methylresorcinarene acts as a reaction vessel/protecting group to control the coordination of copper(II) from cis-see-saw to trans-square planar, and from octahedral to square planar coordination geometry. These processes were studied in solution and in the solid state via1H NMR spectroscopy and single crystal X-ray diffraction.
Introduction
The construction of supramolecular architectures utilizing a variety of weak interactions has potential applications in materials science and biomimetic chemistry.1 The challenge of constructing exotic supramolecular architectures with function from small-molecule building blocks requires a better understanding to design strategies.2 Resorcinarenes are an extensively studied phenolic group containing macrocyclic compounds.3 Easy syntheses, bowl-shape and electron-rich interior cavity are assets strongly associated with resorcinarenes, making them a useful component in host–guest inclusion chemistry.3 The size and the electronic nature of the guest molecules are important for determining the final structures and morphologies of the supramolecular architectures.3 Consequently, different guests have templated assemblies such as open inclusion complexes,4 dimeric and hexameric capsules,5 as well as nanotubes.6The concept of metallosupramolecular chemistry is based on the formation of discrete assemblies or coordination polymers through bridging organic ligands and metals.7 Pyridine N-oxides are typical oxygen atom transfer reagents, routinely used in the syntheses of high-valent transition metal centers, lanthanide and actinide oxo complexes.8 Copper plays an important role in redox chemistry with application in catalysis9 and biology.10 There are multiple reports of different complexes and architectures formed between copper and pyridine N-oxide with applications such as in catalysis,11 as magnetic conducting materials,12 and with cytotoxic characteristics.13
The quest for potential applications of resorcinarenes is a continuous goal for researchers working in this area. There is a need to explore the bowl-shaped interior cavity of electron rich resorcinarenes as an essential feature, treating them as a reaction vessel or a protecting group tuning specific reactions. The aromatic ring of pyridine N-oxides through π⋯π interactions can be bound by the electron-rich resorcinarenes. There are several reports of complexes formed between calix[4]arenes14 and cavitands15 with pyridine N-oxides. Atwood et al.16 reported nano-sized spherical and helical tubular structures formed through hydrophobic and numerous non-covalent interactions, such as metal–ligand coordination, π⋯π stacking, hydrogen bonding, and van der Waals forces associated with p-sulfonatocalix[4]arene, pyridine N-oxide and lanthanide nitrate.
In the study described herein, we explore the electron-rich interior cavity of methylresorcinarene 1 (Fig. 1) as a host for pyridine N-oxides 2–3 to form unique 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand–metal N-oxide CuII square planar products. In the process, methylresorcinarene acts as a protecting group creating steric hindrance for N-oxide coordination, thus changing its coordination mode and the coordination environment of CuII products. These processes were studied in solution and in the solid state via1H NMR spectroscopy and single crystal X-ray diffraction analyses.
1 ligand–metal N-oxide CuII square planar products. In the process, methylresorcinarene acts as a protecting group creating steric hindrance for N-oxide coordination, thus changing its coordination mode and the coordination environment of CuII products. These processes were studied in solution and in the solid state via1H NMR spectroscopy and single crystal X-ray diffraction analyses.
Results and discussion
Resorcinarenes possess an electron rich interior cavity suitable for the recognition of positively charged and electron deficient guest molecules.3–6 In methylresorcinarenes, the electron donating methyl groups increase the electron density of the aromatic rings and thus increase their affinity towards electron deficient guest compounds. The electron push and electron pull nature of the negatively charged oxygen towards the aromatic ring in pyridine N-oxides makes these compounds a unique class of guest molecules, and they act as either electron rich or poor guest molecules with respect to the approaching reagents. Herein, for a π-electron rich receptor, pyridine N-oxide adopts a π-electron deficient system to exhibit π⋯π interactions. Also, the presence of hydroxyl groups in methylresorcinarene and oxygen in pyridine N-oxide makes it a suitable composite for hydrogen bond interactions between the host and the guest. With this prior knowledge of their behaviour towards metal coordination, we started to investigate the host–guest chemistry systematically, starting with solution based studies to inspect such evidence.Host–guest complexation
We recently reported the 1H NMR complexation studies of pyridine N-oxide 2 and methylresorcinarene 1 with an association constant of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 1.8157 ± 0.0171.17 A 1
K = 1.8157 ± 0.0171.17 A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of methylresorcinarene 1 and pyridine N-oxide 2 in CD3OD at 303 K showed significant upfield shifts of the pyridine N-oxide 2 aromatic protons. The most intense shift of 0.62 ppm was observed for the para-protons, thus confirming its location deep in the cavity of the host.17 The generally large shifts of the guest signals highlight the shielding effects of the aromatic rings of the host 1.18
1 mixture of methylresorcinarene 1 and pyridine N-oxide 2 in CD3OD at 303 K showed significant upfield shifts of the pyridine N-oxide 2 aromatic protons. The most intense shift of 0.62 ppm was observed for the para-protons, thus confirming its location deep in the cavity of the host.17 The generally large shifts of the guest signals highlight the shielding effects of the aromatic rings of the host 1.18
The use of the carboxylic acid group at the ortho- position of pyridine N-oxide and its electron withdrawing nature render the aromatic ring further electron deficient. This fact is highlighted by the larger shifts of the 2-picolinic acid N-oxide protons upon complexation with methylresorcinarene 1 (Fig. 2) as compared with the pyridine N-oxide 2. Chemical shift changes greater than 1 ppm are observed for the para- (1.22 ppm) and meta- (1.10 ppm) protons. Again, the large shift of the para-protons also suggests the guest located deep in the cavity of the host.
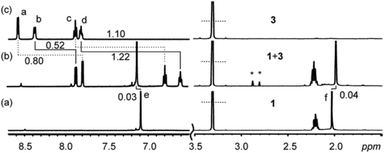 | ||
Fig. 2
IH NMR spectra (CD3OD, 303 K) of a 30 mM solution of (a) 1, (b) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1 and 3, and (c) 3. The chemical shift changes are in ppm. Stars represent water molecules present in the complex (see Fig. 3). 1 mixture of 1 and 3, and (c) 3. The chemical shift changes are in ppm. Stars represent water molecules present in the complex (see Fig. 3). | ||
To further study these systems in the solid state, single crystals suitable for X-ray analysis were obtained by mixing the respective methanol solutions of 1, 2 and 1, 3 to give 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of host 1 + pyridine N-oxide (I) and host 1 + 2-picolinic acid N-oxide (II), respectively, as shown in Fig. 3. Complex I crystallizes in the triclinic space group P
1 complexes of host 1 + pyridine N-oxide (I) and host 1 + 2-picolinic acid N-oxide (II), respectively, as shown in Fig. 3. Complex I crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a 1
with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest ratio, together with three water molecules in the asymmetric unit. Pyridine N-oxide 2 sits inside the cavity at a height of ca. 3.09 Å from the centroid of the lower rim carbon atoms, and stabilizes by π⋯π interactions19 with one of the host aromatic rings at a centroid-to-centroid distance of 3.643 Å. In addition, the meta- and para-hydrogens of pyridine N-oxide 2 are stabilized with C–H⋯π (centroid) interactions at distances of 2.684 Å and 3.040 Å, respectively, as shown in Fig. 3a. The N–O group is a bidentate hydrogen bond acceptor for two out-of-cavity water molecules at (N–O)guest⋯O–H distances of 2.638 Å [∠(N–O)guest⋯O–H, 135.66°] and 2.635 Å [∠(N–O)guest⋯O–H, 163.69°]. The hydrogen bonding between the water molecules and the N–O group of pyridine N-oxide plays an important role in bringing two 1
1 host–guest ratio, together with three water molecules in the asymmetric unit. Pyridine N-oxide 2 sits inside the cavity at a height of ca. 3.09 Å from the centroid of the lower rim carbon atoms, and stabilizes by π⋯π interactions19 with one of the host aromatic rings at a centroid-to-centroid distance of 3.643 Å. In addition, the meta- and para-hydrogens of pyridine N-oxide 2 are stabilized with C–H⋯π (centroid) interactions at distances of 2.684 Å and 3.040 Å, respectively, as shown in Fig. 3a. The N–O group is a bidentate hydrogen bond acceptor for two out-of-cavity water molecules at (N–O)guest⋯O–H distances of 2.638 Å [∠(N–O)guest⋯O–H, 135.66°] and 2.635 Å [∠(N–O)guest⋯O–H, 163.69°]. The hydrogen bonding between the water molecules and the N–O group of pyridine N-oxide plays an important role in bringing two 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest assemblies closer. As a result, the oxygens of N–O groups are at a distance of ca. 6.032 Å, which provided us an insight to glue the N–O groups of the guests with metals (Fig. S3a†).
1 host–guest assemblies closer. As a result, the oxygens of N–O groups are at a distance of ca. 6.032 Å, which provided us an insight to glue the N–O groups of the guests with metals (Fig. S3a†).
The crystal structure of II was solved in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and the asymmetric unit contains a 1
, and the asymmetric unit contains a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex ratio. 2-Picolinic acid N-oxide 3 sits deeper inside the cavity than pyridine N-oxide 2 at a depth of ca. 2.58 Å, stabilized by π⋯π interactions with one of the host aromatic rings at a centroid-to-centroid distance of 3.704 Å. As shown in Fig. 3c, two of the aromatic protons of the guest 3 are stabilized by C–H⋯π interactions at distances of 2.578 Å and 2.658 Å. The N–O group forms an intramolecular hydrogen bond with the –COOH group at a (N–O)guest⋯O–H distance of 2.443(2) Å [∠(N–O)guest⋯O–H, 152(3)°]. However, the intermolecular hydrogen bond with the hydroxyl group of host 1 [(O⋯H–O), 2.748(2) Å; ∠O⋯H–O, 146(3)°] brings two 1
1 host–guest complex ratio. 2-Picolinic acid N-oxide 3 sits deeper inside the cavity than pyridine N-oxide 2 at a depth of ca. 2.58 Å, stabilized by π⋯π interactions with one of the host aromatic rings at a centroid-to-centroid distance of 3.704 Å. As shown in Fig. 3c, two of the aromatic protons of the guest 3 are stabilized by C–H⋯π interactions at distances of 2.578 Å and 2.658 Å. The N–O group forms an intramolecular hydrogen bond with the –COOH group at a (N–O)guest⋯O–H distance of 2.443(2) Å [∠(N–O)guest⋯O–H, 152(3)°]. However, the intermolecular hydrogen bond with the hydroxyl group of host 1 [(O⋯H–O), 2.748(2) Å; ∠O⋯H–O, 146(3)°] brings two 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes together with oxygens of N–O groups at a distance of ca. 3.335 Å (Fig. S3b†).
1 host–guest complexes together with oxygens of N–O groups at a distance of ca. 3.335 Å (Fig. S3b†).
As shown in Fig.3a and b, the C–H⋯π interactions significantly contribute and support the 1H NMR shift changes. The short C–H⋯π distances in II than in I explains the delocalization of shared π-electrons with the electron withdrawing –COOH group, followed by the formation of a stable six-membered ring by intramolecular hydrogen bonding. The large shifts of c- and d-protons of the guest 3 also explains the presence of para- electron withdrawing –COOH and N–O groups, and their shielding by the π-rich cavity after complexation. Besides π⋯π and C–H⋯π interactions, the cavity displays remarkable breathing properties (Fig. S4†) and offers hydrogen bonding with the oxygen atoms of the pyridine N-oxides as well as the solvent molecules. The effect and the strength of shielding on C–H protons by the π-rich cavity also depends upon the height (Fig. S5†) of the guest located in the cavity of the host. With such host breathing properties, guest 3 (2.583 Å) was accessed deeper in the cavity than 2 (3.099 Å), supporting the enhanced shielding observed in solution.
Metal complexation
NMR spectroscopy is a useful tool for studying the structural and magnetic properties of CuII coordination compounds.20 The slow electronic relaxation of CuII ions mostly results in large line widths and poor resolution, making the interpretation of spectra of CuII complexes almost impossible. This paramagnetic effect is stronger for protons in close proximity to the copper ions.20The 1H NMR spectra of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of pyridine N-oxide 2 and CuCl2 show only one broad signal around 9.2 ppm within the 0–100 ppm window (Fig. 4c). From X-ray crystal structures (Fig. 3), the guest is tilted towards a phenyl ring of the host 1 to maximize π⋯π interactions. This orientation of the pyridine N-oxide creates steric hindrance for bidentate coordination, which will tune the coordination geometry of the CuII.
1 mixture of pyridine N-oxide 2 and CuCl2 show only one broad signal around 9.2 ppm within the 0–100 ppm window (Fig. 4c). From X-ray crystal structures (Fig. 3), the guest is tilted towards a phenyl ring of the host 1 to maximize π⋯π interactions. This orientation of the pyridine N-oxide creates steric hindrance for bidentate coordination, which will tune the coordination geometry of the CuII.
 | ||
Fig. 4
IH NMR spectra (CD3OD, 303 K) of a 30 mM solution of (a) 1, (b) a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1, 2 and CuCl2, (c) a 1 1 mixture of 1, 2 and CuCl2, (c) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2 and CuCl2, and (d) 2. The shift changes are highlighted by the dotted lines. Stars represent water molecules present in the complex (see Fig. 5). 1 mixture of 2 and CuCl2, and (d) 2. The shift changes are highlighted by the dotted lines. Stars represent water molecules present in the complex (see Fig. 5). | ||
A series of 1H NMR experiments were done to test this hypothesis. In the experiment, several samples were prepared consisting of the host 1, the N-oxides 2–3, CuCl2 and Cu(NO3)2·3H2O salts. The 1H NMR spectrum of the mixture containing 1, 2 and CuCl2 shows a substantial increase of 0.84 ppm of the broad pyridine N-oxide signals at 8.3 ppm (Fig. 4b). This upfield shift is either consistent with shielding of the guest signals by the phenyl rings of the host 1 or the formation of a different product. The pyridine N-oxide 2 signals are broadened as a result of the slow relaxation of the CuII ions, while all the methylresorcinarene 1 signals are observed. The upfield shifts of the methylresorcinarene 1 signals support the formation of a host–guest complex with the CuII coordinated pyridine N-oxides (Fig. 4).
The 1H NMR spectrum of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2-picolinic acid N-oxide 3 and Cu(NO3)2·3H2O was analogous to the pyridine N-oxide 2, with a single broad signal around 10.5 ppm within the 0–100 ppm window (Fig. S6†). This larger downfield shift is as a result of the more electron-deficient product. The single broad signal of the 2-picolinic acid N-oxide 3 disappears in the combination of 1, 3 and Cu(NO3)2·3H2O, also suggesting shielding or the formation of a different product. However, upfield changes of the methylresorcinarene 1 signals are analogous to those observed with the pyridine N-oxide 2, hinting at a similar host–guest product (Fig. S6†).
1 mixture of 2-picolinic acid N-oxide 3 and Cu(NO3)2·3H2O was analogous to the pyridine N-oxide 2, with a single broad signal around 10.5 ppm within the 0–100 ppm window (Fig. S6†). This larger downfield shift is as a result of the more electron-deficient product. The single broad signal of the 2-picolinic acid N-oxide 3 disappears in the combination of 1, 3 and Cu(NO3)2·3H2O, also suggesting shielding or the formation of a different product. However, upfield changes of the methylresorcinarene 1 signals are analogous to those observed with the pyridine N-oxide 2, hinting at a similar host–guest product (Fig. S6†).
To unambiguously confirm the structures of the host–ligand–metal complexes, solid state analyses via single crystal X-ray diffraction were done. Reactions of pyridine N-oxide 2 and CuCl2 and between 2-picolinic acid N-oxide 3 and Cu(NO3)2·3H2O resulted in a discrete structure III (Fig. 5a) and 1D polymeric self-assembly V (Fig. 5d), respectively. Complex III crystallized in the monoclinic space group P21/c with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to metal ratio. The μ2-O,O pyridine N-oxide 2 bridges Cu1 and Cu1a, with CuII in the Cl2O2 coordination sphere, and have adopted cis-see-saw III21 (τ4 = 0.34)22 geometry.
1 ligand to metal ratio. The μ2-O,O pyridine N-oxide 2 bridges Cu1 and Cu1a, with CuII in the Cl2O2 coordination sphere, and have adopted cis-see-saw III21 (τ4 = 0.34)22 geometry.
On the other hand, complex V is a 1D polymeric structure (Fig. 5d and S9†) with octahedral CuII in the O4 coordination sphere. Complex V crystallized in the monoclinic space group P21/c, the asymmetric unit contains one 2-picolinic acid N-oxide 3 chelating half a copper in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to metal ratio. A CCDC search related to III (CSD Refcodes: CUCPYO, CUCPYO11 and CUCPYO12)21 and V (CSD Refcode: SIJRIN)23 revealed three and one hits, respectively, which are synthesized under different conditions.
1 ligand to metal ratio. A CCDC search related to III (CSD Refcodes: CUCPYO, CUCPYO11 and CUCPYO12)21 and V (CSD Refcode: SIJRIN)23 revealed three and one hits, respectively, which are synthesized under different conditions.
Single crystal X-ray structure from the combination of the host 1, the N-oxides 2–3 and the CuII salts CuCl2 and Cu(NO3)2·3H2O gave the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–ligand–metal products of (1)2 + (2)2 + CuCl2, IV (Fig. 5b and c) and (1)2 + ([3–2H]−2)2 + Cu2+, VI, respectively (Fig. 5e and f). Interestingly, the coordination geometry of the CuII is different from the products obtained without host 1. The reaction of 2 + CuCl2 and 3 + Cu(NO3)2·3H2O with methylresorcinarene 1 retains the characteristic π⋯π interactions (IV; 3.956 Å and VI; 3.869 Å) as shown in Fig. 5b and e. Although both structures IV and VI show similar interactions, the 2-picolinic acid N-oxide 3 in VI is located deeper (2.643 Å) in the cavity of the host 1 compared with pyridine N-oxide 2 in IV (3.099 Å), thus displaying shorter C–H⋯π interactions. In complex IV, the CuII is trans-coordinated by two chloride anions and pyridine N-oxide 2 molecules (Fig. 5b), while in complex VI, 2-picolinic acid N-oxide 3 alone chelates in trans-mode with the help of deprotonated –COOH functionality (Fig. 5c). The self-assembly of the trans-coordination mode between pyridine N-oxide and CuCl2 in complex IV has not been previously reported. A CSD survey revealed 10 crystal structures with other N-oxides and CuCl2 having a similar trans-coordination mode (Refcodes: CEGGOK, CMPOCU, CMPOCU01, DETFAK, IVVUYUU, PIJDUH, QQQBVY, QQQBWA, TANSUW and TANSUW10).24 On the other hand, the four coordinated trans-chelation mode between deprotonated 2-picolinic acid N-oxide and metals of M(NO3)2 stabilized with solvent molecules apically is a commonly observed phenomenon (for example, see Refcodes: BIVWIM, BIVWOS, BIVWUY, BIVXAF, IDULOJ, TENKAA, XISBOR, TOTTED, TOZMEC, TOZMEC01).25 The four coordinated and trans-chelated complex inside the complex VI stabilized by the resorcinarene host is rare, and a CSD search for similar four coordinate systems revealed one hit (Refcode: EBUPIC).26 Besides different coordination spheres of CuII and their stabilization by hydrogen bond interactions, N-oxides 2–3 and methylresorcinarene components prefer to exchange the π-electrons by π⋯π and C–H⋯π interactions.
1 host–ligand–metal products of (1)2 + (2)2 + CuCl2, IV (Fig. 5b and c) and (1)2 + ([3–2H]−2)2 + Cu2+, VI, respectively (Fig. 5e and f). Interestingly, the coordination geometry of the CuII is different from the products obtained without host 1. The reaction of 2 + CuCl2 and 3 + Cu(NO3)2·3H2O with methylresorcinarene 1 retains the characteristic π⋯π interactions (IV; 3.956 Å and VI; 3.869 Å) as shown in Fig. 5b and e. Although both structures IV and VI show similar interactions, the 2-picolinic acid N-oxide 3 in VI is located deeper (2.643 Å) in the cavity of the host 1 compared with pyridine N-oxide 2 in IV (3.099 Å), thus displaying shorter C–H⋯π interactions. In complex IV, the CuII is trans-coordinated by two chloride anions and pyridine N-oxide 2 molecules (Fig. 5b), while in complex VI, 2-picolinic acid N-oxide 3 alone chelates in trans-mode with the help of deprotonated –COOH functionality (Fig. 5c). The self-assembly of the trans-coordination mode between pyridine N-oxide and CuCl2 in complex IV has not been previously reported. A CSD survey revealed 10 crystal structures with other N-oxides and CuCl2 having a similar trans-coordination mode (Refcodes: CEGGOK, CMPOCU, CMPOCU01, DETFAK, IVVUYUU, PIJDUH, QQQBVY, QQQBWA, TANSUW and TANSUW10).24 On the other hand, the four coordinated trans-chelation mode between deprotonated 2-picolinic acid N-oxide and metals of M(NO3)2 stabilized with solvent molecules apically is a commonly observed phenomenon (for example, see Refcodes: BIVWIM, BIVWOS, BIVWUY, BIVXAF, IDULOJ, TENKAA, XISBOR, TOTTED, TOZMEC, TOZMEC01).25 The four coordinated and trans-chelated complex inside the complex VI stabilized by the resorcinarene host is rare, and a CSD search for similar four coordinate systems revealed one hit (Refcode: EBUPIC).26 Besides different coordination spheres of CuII and their stabilization by hydrogen bond interactions, N-oxides 2–3 and methylresorcinarene components prefer to exchange the π-electrons by π⋯π and C–H⋯π interactions.
The π⋯π and C–H⋯π interactions between the host 1 and the guest molecules 2, 3 engendered a steric effect, thus causing a dramatic change in the coordination sphere around CuII, which is different from complexes III and V.20,22 As a consequence, the bidentate pyridine N-oxide 2 in the L2M2 host free complex now adopts a monodentate coordination mode with trans-L2M geometry in IV (Fig. 5). The coordination sphere of the CuII changes from cis-see-saw in III to trans-square planar geometry in IV. The coordination sphere of square planar CuII in IV is tightly held and stabilized by –OH⋯O (2.803 Å, ∠O–H⋯O 138.57°) and –OH⋯Cl (3.127 Å, ∠O–H⋯Cl 163.33°) interactions (Fig. S7b†). Interestingly, the N-oxide in IV preserves its bidenticity through hydrogen bonding with a methanol molecule (Fig. S7b†). The CuII in VI is apically stabilized by water molecules at a Cu⋯O distance of 2.740 Å. Square planar geometries, especially CuII ions, compete with aqua ligands for binding and such a preference often leads to ligand field stabilization, either by strong coordination24 or by weak interactions (Fig. S8b†).
Conclusions
In summary, the interior cavity of methylresorcinarene 1 through π⋯π, CH⋯π and hydrogen bond interactions templates the formation of a unique 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (host–ligand–metal) complex with N-oxides 2–3 and CuII salts (CuCl2 and Cu(NO3)2·3H2O). The coordination geometry of the CuII changes from cis-see-saw (III) to trans-square planar (IV), and from octahedral (V) to square planar (VI) products. With pyridine N-oxide 2, the anion (Cl−) completes the coordination geometry. Introducing a chelating carboxylic acid functional group in the ortho-position of the pyridine N-oxide 3 led to a similar coordination compound. Though the CuII ion retains the same geometry, the carboxylic acid group completes the coordination geometry with the nitrate anion as a passive spectator. Despite the paramagnetic nature of CuII, the host signals could be monitored to confirm the complexation in solution via1H NMR spectroscopy. Single crystal X-ray diffraction studies unambiguously confirmed the formed products and their specific coordination geometries. This work highlights the usefulness of the resorcinarene framework as a reaction vessel for pyridine N-oxide copper complexes in tuning specific CuII coordination sphere products governed by several weak interactions.
1 (host–ligand–metal) complex with N-oxides 2–3 and CuII salts (CuCl2 and Cu(NO3)2·3H2O). The coordination geometry of the CuII changes from cis-see-saw (III) to trans-square planar (IV), and from octahedral (V) to square planar (VI) products. With pyridine N-oxide 2, the anion (Cl−) completes the coordination geometry. Introducing a chelating carboxylic acid functional group in the ortho-position of the pyridine N-oxide 3 led to a similar coordination compound. Though the CuII ion retains the same geometry, the carboxylic acid group completes the coordination geometry with the nitrate anion as a passive spectator. Despite the paramagnetic nature of CuII, the host signals could be monitored to confirm the complexation in solution via1H NMR spectroscopy. Single crystal X-ray diffraction studies unambiguously confirmed the formed products and their specific coordination geometries. This work highlights the usefulness of the resorcinarene framework as a reaction vessel for pyridine N-oxide copper complexes in tuning specific CuII coordination sphere products governed by several weak interactions.
Acknowledgements
We gratefully acknowledge the Academy of Finland (N.K.B.; grant no. 258653) and the University of Jyvaskyla for financial support. Prof. Kari Rissanen and Dr Sandip Bhowmik are acknowledged for help in data interpretation.Notes and references
- (a) R. H. Vreekamp, J. P. M. van Duynhoven, M. Hubert, W. Verboom and D. N. Reinhoudt, Angew. Chem., Int. Ed. Engl., 1996, 35, 1215–1218 CrossRef CAS PubMed; (b) S. Mann, Biomimetic Materials Chemistry, Wiley VCH Verlag GmbH, 1996 Search PubMed; (c) A. Firouzi, D. Kumar, L. M. Bull, T. Besier, P. Sieger, Q. Huo, S. A. Walker, J. A. Zasadzinski, C. Glinka, J. Nicol, D. Marolese, G. D. Sturky and B. F. Chmelka, Science, 1995, 267, 1138–1143 CrossRef CAS; (d) C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS; (e) H. L. Huang, K. E. Wooley and E. Remsen, Chem. Commun., 1998, 1415–1416 RSC; (f) R. S. Meissner, J. Rebek and J. de Mendoza, Science, 1995, 270, 1485–1488 CAS; (g) K. D. Shimizu and J. Rebek, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 12403–12407 CrossRef CAS.
- (a) L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469–472 CrossRef CAS PubMed; (b) V. S. K. Balagurusamy, G. Ungar, V. Percec and G. Johansson, J. Am. Chem. Soc., 1997, 119, 1539–1555 CrossRef CAS; (c) N. Khazanovich, J. R. Granja, D. E. McRee, R. A. Milligan and M. R. Ghadiri, J. Am. Chem. Soc., 1994, 116, 6011–6012 CrossRef CAS.
- (a) P. Timmerman, W. Verboom and D. N. Reinhoudt, Tetrahedron, 1996, 52, 2663–2704 CrossRef CAS; (b) A. Jasat and J. C. Sherman, Chem. Rev., 1999, 99, 931–968 CrossRef CAS PubMed.
- (a) D. M. Rudkevich and J. Rebek, Eur. J. Org. Chem., 1999, 1991–2005 CrossRef CAS; (b) M. Nissinen and K. Rissanen, Supramol. Chem., 2003, 15, 581–590 CrossRef CAS PubMed.
- (a) M. Luostarinen, A. Åhman, M. Nissinen and K. Rissanen, Supramol. Chem., 2004, 16, 505–512 CrossRef CAS PubMed; (b) H. Mansikkamäki, C. A. Schalley, M. Nissinen and K. Rissanen, New J. Chem., 2005, 29, 116–127 RSC; (c) A. Shivanyuk and J. Rebek, J. Am. Chem. Soc., 2003, 125, 3432–3433 CrossRef CAS PubMed; (d) J. L. Atwood, L. J. Barbour and A. Jerga, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4837–4841 CrossRef CAS PubMed; (e) N. K. Beyeh, M. Kogej, A. Åhman, K. Rissanen and C. A. Schalley, Angew. Chem., Int. Ed., 2006, 45, 5214–5218 CrossRef CAS PubMed.
- H. Mansikkamäki, M. Nissinen and K. Rissanen, Angew. Chem., Int. Ed., 2004, 43, 1243–1246 CrossRef PubMed.
- (a) J. W. Steed and J. L. Atwood, in Supramolecular Chemistry, John Wiley and Sons, Ltd, 2009, pp. 591–706 Search PubMed; (b) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313–323 CrossRef CAS PubMed; (c) M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J.-M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3644–3662 CrossRef CAS PubMed; (d) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703–6712 RSC; (e) M. A. Halcrow, Dalton Trans., 2009, 2059–2073 RSC; (f) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2012, 113, 734–777 CrossRef PubMed; (g) M. D. Ward and P. R. Raithby, Chem. Soc. Rev., 2013, 42, 1619–1636 RSC.
- (a) R. R. Schrock, Chem. Rev., 2001, 102, 145–180 CrossRef PubMed; (b) C. C. Cummins, R. R. Schrock and W. M. Davis, Inorg. Chem., 1994, 33, 1448–1457 CrossRef CAS; (c) S. M. Mullins, A. P. Duncan, R. G. Bergman and J. Arnold, Inorg. Chem., 2001, 40, 6952–6963 CrossRef CAS PubMed; (d) K.-M. Sung and R. H. Holm, J. Am. Chem. Soc., 2001, 123, 1931–1943 CrossRef CAS PubMed; (e) J. A. Pool, B. L. Scott and J. L. Kiplinger, J. Am. Chem. Soc., 2005, 127, 1338–1339 CrossRef CAS PubMed; (f) D. S. J. Arney and C. J. Burns, J. Am. Chem. Soc., 1995, 117, 9448–9460 CrossRef CAS; (g) J. Jia, A. J. Blake, N. R. Champness, P. Hubberstey, C. Wilson and M. Schröder, Inorg. Chem., 2008, 47, 8652–8664 CrossRef CAS PubMed; (h) A. E. V. Gorden, J. Xu, K. N. Raymond and P. Durbin, Chem. Rev., 2003, 103, 4207–4282 CrossRef CAS PubMed.
- (a) T. Punniyamurthy and L. Rout, Coord. Chem. Rev., 2008, 252, 134–154 CrossRef CAS PubMed; (b) G. Battaini, A. Granata, E. Monzani, M. Gullotti and L. Casella, Adv. Inorg. Chem., 2006, 58, 185–233 CrossRef CAS.
- (a) E. I. Solomon, R. Sarangi, J. S. Woertink, A. J. Augustine, J. Yoon and S. Ghosh, Acc. Chem. Res., 2007, 40, 581–591 CrossRef CAS PubMed; (b) E. I. Solomon, P. Chen, M. Metz, S.-K. Lee and A. E. Palmer, Angew. Chem., Int. Ed., 2001, 40, 4570–4590 CrossRef CAS; (c) J. P. Klinman, Chem. Rev., 1996, 96, 2541–2562 CrossRef CAS PubMed.
- A. Livieri, M. Boiocchi, G. Desimoni and G. Faita, Chem. – Eur. J., 2011, 17, 516–520 CrossRef CAS PubMed.
- (a) F. Pointillart, T. Cauchy, Y. Le Gal, S. Golhen, O. Cador and L. Ouahab, Chem. Commun., 2010, 46, 4947–4949 RSC; (b) J.-G. Lin, Y. Su, Z.-F. Tian, L. Qiu, L.-L. Wen, Z.-D. Lu, Y.-Z. Li and Q.-J. Meng, Cryst. Growth Des., 2007, 7, 2526–2534 CrossRef CAS; (c) J.-M. Shi, Y.-M. Sun, Z. Liu, L.-D. Liu, W. Shi and P. Cheng, Dalton Trans., 2006, 376–380 RSC.
- (a) A. Puszko, L. Wasylina, M. Pełczynska, Z. Staszak, A. Adach, M. Cieślak-Golonka and M. Kubiak, J. Inorg. Biochem., 2007, 101, 117–126 CrossRef CAS PubMed; (b) A. Puszko, A. Brzuszkiewicz, J. Jezierska, A. Adach, J. Wietrzyk, B. Filip, M. Pełczynska and M. Cieslak-Golonka, J. Inorg. Biochem., 2011, 105, 1109–1114 CrossRef CAS PubMed.
- G. Zheng, Y.-Y. Li, H.-D. Guo, S.-Y. Song and H.-J. Zhang, Chem. Commun., 2008, 4918–4920 RSC.
- (a) L. Adriaenssens and P. Ballester, Chem. Soc. Rev., 2013, 42, 3261–3277 RSC; (b) A. Galán, E. C. Escudero-Adán, A. Frontera and P. Ballester, J. Org. Chem., 2014, 79, 5545–5557 CrossRef PubMed.
- G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049–1052 CrossRef CAS.
- N. K. Beyeh, R. Puttreddy and K. Rissanen, RSC Adv., 2015, 5, 30222–30226 RSC.
- T. Helgaker, M. Jaszunski and K. Ruud, Chem. Rev., 1999, 99, 293–352 CrossRef CAS PubMed.
- G. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885–3896 RSC.
- (a) R. C. Holz and J. M. Brink, Inorg. Chem., 1994, 33, 4609–4610 CrossRef CAS; (b) N. N. Murthy, K. D. Karlin, I. Bertini and C. Luchinat, J. Am. Chem. Soc., 1997, 119, 2156–2162 CrossRef CAS; (c) J. M. Brink, R. A. Rose and R. C. Holz, Inorg. Chem., 1996, 35, 2878–2885 CrossRef CAS; (d) G. Aromí, P. Gamez, H. Kooijman, A. L. Spek, W. L. Driessen and J. Reedijk, Eur. J. Inorg. Chem., 2003, 2003, 1394–1400 CrossRef PubMed.
- (a) R. S. Sager, R. J. Williams and W. H. Watson, Inorg. Chem., 1967, 6, 951–955 CrossRef CAS; (b) H. L. Schäfer, J. C. Morrow and H. M. Smith, J. Chem. Phys., 1965, 42, 504–508 CrossRef PubMed; (c) A. M. Atria, P. Cortes, M. T. Garland and R. Baggio, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2003, 59, 967–969 Search PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- W.-P. Wu, Y.-Y. Wang, Y.-P. Wu, J.-Q. Liu, X.-R. Zeng, Q.-Z. Shi and S.-M. Peng, CrystEngComm, 2007, 9, 753–757 RSC.
- (a) W. H. Watson and D. R. Johnson, Inorg. Chem., 1971, 10, 1068–1072 CrossRef CAS; (b) M. R. Kidd, R. S. Sager and W. H. Watson, Inorg. Chem., 1967, 6, 946–951 CrossRef CAS; (c) A. Puszko, L. Wasylina, M. Pelczynska, Z. Staszak, A. Adach, M. Cieslak-Golonka and M. Kubiak, J. Inorg. Biochem., 2007, 101, 117–126 CrossRef CAS PubMed; (d) V. B. Rybakov, T. A. Semenova, L. A. Aleshina, V. P. Andreev, Y. P. Nizhnik and V. V. Chernyshev, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004, 60, 901–903 Search PubMed; (e) J. Kozisek, P. Baran and D. Valigura, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1992, 48, 31–33 CrossRef; (f) P. Knuuttila, Acta Chem. Scand., 1983, 37, 765–769 CrossRef PubMed; (g) P. Knuuttila, Acta Chem., Scand. Ser. A, 1982, 36, 767–772 CrossRef PubMed.
- (a) X.-B. Li, R.-L. Shang and B.-W. Sun, Acta. Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, 131 Search PubMed; (b) Z. Hnatejko, G. Dutkiewicz, M. Kubicki and S. Lis, J. Mol. Struct., 2013, 1034, 128–133 CrossRef CAS PubMed; (c) Y.-M. Liu, Y.-Y. Xu, J.-G. Lin, F.-M. Wang, C.-S. Lu and Q.-J. Meng, Inorg. Chem. Commun., 2010, 13, 689–693 CrossRef CAS PubMed; (d) J. Chen, Y. Lu, W.-S. Wu, J.-C. Dai and J.-M. Lin, Acta. Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, 1540–1541 Search PubMed.
- Q. Gao, Y.-B. Xie, M. Thorstad, J.-H. Sun, Y. Cui and H.-C. Zhou, CrystEngComm, 2011, 13, 6787–6793 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic and NMR spectroscopic data. CCDC 1054267–1054272. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt01143d |
| This journal is © The Royal Society of Chemistry 2015 |



