Synthesis, characterization, and atropisomerism of iron complexes containing the tetrakis(2-chloro-6-fluorophenyl)porphyrinate ligand†
Abstract
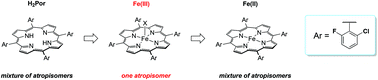
Iron complexes of the meso-(tetraaryl)porphyrin, 5,10,15,20-tetrakis(2-chloro-6-fluorophenyl)porphine (H2ClFTPP) are reported. This unique ligand affords the opportunity to study atropisomerism in a porphyrin system containing similar substituents in the 2 and 6 positions of the meso-aryl rings. The atropisomerism displayed by the iron porphyrinates is observed to be a function of both the oxidation state of the metal and the nature of the axial ligand. In the case of iron(III) porphyrinates, a single atropisomer is favored, whereas with the iron(II) porphyrinate a statistical distribution of all possible atropisomers is observed. Variable temperature studies with the iron(II) porphyrinate demonstrate that the distribution of atropisomers is maintained even at elevated temperatures. The results are discussed in the context of atropisomerism with other meso-(tetraaryl)porphyrins.

 Please wait while we load your content...
Please wait while we load your content...