Open Access Article
Open Access ArticleA thorium metallacyclopentadiene complex: a combined experimental and computational study†
Bo
Fang
a,
Guohua
Hou
a,
Guofu
Zi
*a,
De-Cai
Fang
*a and
Marc D.
Walter
*b
aDepartment of Chemistry, Beijing Normal University, Beijing 100875, China. E-mail: gzi@bnu.edu.cn; dcfang@bnu.edu.cn; Fax: +86-10-58802075; Tel: +86-10-58806051
bInstitut für Anorganische und Analytische Chemie, Technische Universität Braunschweig, Hagenring 30, 38106 Braunschweig, Germany. E-mail: mwalter@tu-bs.de; Fax: +49-531-3915387; Tel: +49-531-3915312
First published on 23rd March 2015
Abstract
The synthesis, structure, and reactivity of a thorium metallacyclopentadiene were comprehensively studied. The reduction of (η5-C5Me5)2ThCl2 (1) with potassium graphite (KC8) in the presence of diphenylacetylene (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh) yields the first thorium metallacyclopentadiene complex (η5-C5Me5)2Th(η2-C4Ph4) (2). Density functional theory (DFT) studies suggest that the 5f orbitals are involved in the bonding of the metallacyclopentadiene Th–(η2-C
CPh) yields the first thorium metallacyclopentadiene complex (η5-C5Me5)2Th(η2-C4Ph4) (2). Density functional theory (DFT) studies suggest that the 5f orbitals are involved in the bonding of the metallacyclopentadiene Th–(η2-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
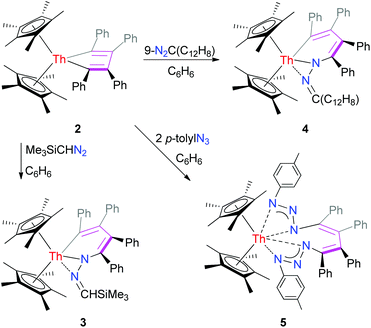
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) moiety. Compared to the thorium metallacyclopropene, complex 2 shows a distinctively different reactivity towards diazoalkanes and organic azides such as Me3SiCHN2, 9-diazofluorene and p-tolylN3, leading to the formation of the six-membered hydrazido complexes (η5-C5Me5)2Th[N(N
C) moiety. Compared to the thorium metallacyclopropene, complex 2 shows a distinctively different reactivity towards diazoalkanes and organic azides such as Me3SiCHN2, 9-diazofluorene and p-tolylN3, leading to the formation of the six-membered hydrazido complexes (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSiMe3)(C4Ph4)] (3) and (η5-C5Me5)2Th[N(N
CHSiMe3)(C4Ph4)] (3) and (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C12H8))(C4Ph4)] (4) and the seven-membered bis(triazenido) complex (η5-C5Me5)2Th[N(N
C(C12H8))(C4Ph4)] (4) and the seven-membered bis(triazenido) complex (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))(C4Ph4)N(N
N(p-tolyl))(C4Ph4)N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))] (5), respectively.
N(p-tolyl))] (5), respectively.
Introduction
Small metallacycles exhibit unusual intrinsic reactivity,1 and within this class of compounds metallacyclopropenes and metallacyclopentadienes of group 4 metallocenes have spurred particular interest.1,2 These metallacycles are generally prepared by the reduction of the metallocene dichlorides in the presence of a suitable alkyne.1c Whereas the synthesis of metallacyclopropenes requires a precise control of the alkyne stoichiometry, metallacyclopentadienes are formed in the presence of an excess alkyne.1c Furthermore, metallacycles may serve as precursors for the synthesis of highly functionalized organic molecules and heterocyclic main group element compounds.1–3 Whereas metallacycles of the group 4 elements are well studied and understood, only a few examples of actinide elements are known.4 One notable exception is the reactivity of the (η5-C5Me5)2U moiety with PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh to yield the uranium metallacyclopentadiene (η5-C5Me5)2U(η5-C4Ph4), which most likely proceeds via the uranium metallacyclopropene intermediate (η5-C5Me5)2U(PhC
CPh to yield the uranium metallacyclopentadiene (η5-C5Me5)2U(η5-C4Ph4), which most likely proceeds via the uranium metallacyclopropene intermediate (η5-C5Me5)2U(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh).4e In contrast, the analogous reactivity of the (η5-C5Me5)2Th fragment has not been investigated. We have been interested in thorium organometallics for some time,5–8 because Th adopts with its 7s26d2 electronic ground state, a special position within the actinide elements, which also relates it to group 4 metals. In order to investigate the reaction chemistry of thorium metallacycles and the influence of the 5f-orbitals we have recently prepared the thorium metallacyclopropene complex [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2).9 The sterically demanding 1,2,4-(Me3C)3C5H2 ligand prevented the double insertion of PhC
CPh).4e In contrast, the analogous reactivity of the (η5-C5Me5)2Th fragment has not been investigated. We have been interested in thorium organometallics for some time,5–8 because Th adopts with its 7s26d2 electronic ground state, a special position within the actinide elements, which also relates it to group 4 metals. In order to investigate the reaction chemistry of thorium metallacycles and the influence of the 5f-orbitals we have recently prepared the thorium metallacyclopropene complex [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2).9 The sterically demanding 1,2,4-(Me3C)3C5H2 ligand prevented the double insertion of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh and therefore it allowed us to investigate the reactivity of the thorium metallacyclopropene towards unsaturated substrates such as aldehyde, CS2, carbodiimide, nitrile, and isothiocyanate, for which insertion into the Th–C bond was observed.9 With the organic azide Me3SiN3 the azametallacyclobutene complex [η5-1,2,4-(Me3C)3C5H2]2Th[N(SiMe3)C(Ph)
CPh and therefore it allowed us to investigate the reactivity of the thorium metallacyclopropene towards unsaturated substrates such as aldehyde, CS2, carbodiimide, nitrile, and isothiocyanate, for which insertion into the Th–C bond was observed.9 With the organic azide Me3SiN3 the azametallacyclobutene complex [η5-1,2,4-(Me3C)3C5H2]2Th[N(SiMe3)C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)] was formed concomitant with N2 loss, whereas the unusual complex [η5-1,2,4-(Me3C)3C5H2][η5,σ-1,2-(Me3C)2-4-(CH2CMe2)C5H2]Th[NC(C12H8)CH(Ph)C(Ph)
C(Ph)] was formed concomitant with N2 loss, whereas the unusual complex [η5-1,2,4-(Me3C)3C5H2][η5,σ-1,2-(Me3C)2-4-(CH2CMe2)C5H2]Th[NC(C12H8)CH(Ph)C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N] was isolated when [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2) was treated with 9-diazofluorene (Scheme 1).9 We are now interested in comparing the reactivity of a thorium metallacyclopentadiene towards these reactive nitrogen containing substrates.10 Therefore, as part of these investigations, we report herein on some observations concerning the synthesis, structure, structure–reactivity relationship of the first thorium metallacyclopentadiene (η5-C5Me5)2Th(η2-C4Ph4) (2), and its reactivity towards organic azide and diazoalkane derivatives.
N] was isolated when [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2) was treated with 9-diazofluorene (Scheme 1).9 We are now interested in comparing the reactivity of a thorium metallacyclopentadiene towards these reactive nitrogen containing substrates.10 Therefore, as part of these investigations, we report herein on some observations concerning the synthesis, structure, structure–reactivity relationship of the first thorium metallacyclopentadiene (η5-C5Me5)2Th(η2-C4Ph4) (2), and its reactivity towards organic azide and diazoalkane derivatives.
Experimental
General methods
All reactions and product manipulations were carried out under an atmosphere of dry dinitrogen with rigid exclusion of air and moisture using standard Schlenk or cannula techniques, or in a glove box. All organic solvents were freshly distilled from sodium benzophenone ketyl immediately prior to use. Diphenylacetylene was purified by sublimation prior to use. KC8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and 9-diazofluorene12 were prepared according to literature procedures. All other chemicals were purchased from Aldrich Chemical Co. and Beijing Chemical Co. and used as received unless otherwise noted. Infrared spectra were recorded in KBr pellets on an Avatar 360 Fourier transform spectrometer. 1H and 13C{1H} NMR spectra were recorded on a Bruker AV 400 spectrometer at 400 and 100 MHz, respectively. All chemical shifts are reported in δ units with reference to the residual protons of the deuterated solvents, which served as internal standards, for proton and carbon chemical shifts. Melting points were measured on an X-6 melting point apparatus and were uncorrected. Elemental analyses were performed on a Vario EL elemental analyzer.
11 and 9-diazofluorene12 were prepared according to literature procedures. All other chemicals were purchased from Aldrich Chemical Co. and Beijing Chemical Co. and used as received unless otherwise noted. Infrared spectra were recorded in KBr pellets on an Avatar 360 Fourier transform spectrometer. 1H and 13C{1H} NMR spectra were recorded on a Bruker AV 400 spectrometer at 400 and 100 MHz, respectively. All chemical shifts are reported in δ units with reference to the residual protons of the deuterated solvents, which served as internal standards, for proton and carbon chemical shifts. Melting points were measured on an X-6 melting point apparatus and were uncorrected. Elemental analyses were performed on a Vario EL elemental analyzer.
Syntheses
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CHSiMe3)(C4Ph4)]·0.5C6H6 (3·0.5C6H6).
Method A. An n-hexane (125 μL) solution of Me3SiCHN2 (0.25 mmol, 2 M in n-hexane) was added dropwise to a toluene (10 mL) solution of (η5-C5Me5)2Th(η2-C4Ph4) (2; 215 mg, 0.25 mmol) with stirring at room temperature. During the course of the reaction, the color of the solution changed from yellow to red. After the solution was stirred at room temperature overnight, the solvent was removed. The residue was extracted with benzene (10 mL × 3) and filtered. The volume of the filtrate was reduced to 2 mL, red crystals of 3·0.5C6H6 were isolated, when this solution was kept at room temperature for one week. Yield: 217 mg (86%) (found: C, 65.32; H, 6.21; N, 2.83. C55H63N2SiTh requires C, 65.26; H, 6.27; N, 2.77%). M.p.: 138–140 °C (decomp.). 1H NMR (C6D6): δ 7.15 (s, 3H, C6H6), 7.14 (s, 1H, NCHSi), 7.10 (t, J = 7.5 Hz, 4H, phenyl), 7.00 (m, 4H, phenyl), 6.90 (t, J = 7.4 Hz, 2H, phenyl), 6.83 (m, 6H, phenyl), 6.74 (t, J = 7.5 Hz, 2H, phenyl), 6.66 (m, 2H, phenyl), 2.04 (s, 30H, CH3), 0.12 (s, 9H, Si(CH3)3) ppm. 13C{1H} NMR (C6D6): δ 215.2 (ThCPh), 149.7 (C
CHSiMe3)(C4Ph4)]·0.5C6H6 (3·0.5C6H6).
Method A. An n-hexane (125 μL) solution of Me3SiCHN2 (0.25 mmol, 2 M in n-hexane) was added dropwise to a toluene (10 mL) solution of (η5-C5Me5)2Th(η2-C4Ph4) (2; 215 mg, 0.25 mmol) with stirring at room temperature. During the course of the reaction, the color of the solution changed from yellow to red. After the solution was stirred at room temperature overnight, the solvent was removed. The residue was extracted with benzene (10 mL × 3) and filtered. The volume of the filtrate was reduced to 2 mL, red crystals of 3·0.5C6H6 were isolated, when this solution was kept at room temperature for one week. Yield: 217 mg (86%) (found: C, 65.32; H, 6.21; N, 2.83. C55H63N2SiTh requires C, 65.26; H, 6.27; N, 2.77%). M.p.: 138–140 °C (decomp.). 1H NMR (C6D6): δ 7.15 (s, 3H, C6H6), 7.14 (s, 1H, NCHSi), 7.10 (t, J = 7.5 Hz, 4H, phenyl), 7.00 (m, 4H, phenyl), 6.90 (t, J = 7.4 Hz, 2H, phenyl), 6.83 (m, 6H, phenyl), 6.74 (t, J = 7.5 Hz, 2H, phenyl), 6.66 (m, 2H, phenyl), 2.04 (s, 30H, CH3), 0.12 (s, 9H, Si(CH3)3) ppm. 13C{1H} NMR (C6D6): δ 215.2 (ThCPh), 149.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 148.1 (CPh), 147.2 (CPh), 146.8 (CPh), 143.2 (phenyl C), 140.0 (phenyl C), 135.3 (phenyl C), 133.8 (phenyl C), 132.7 (phenyl C), 130.4 (phenyl C), 129.5 (phenyl C), 128.9 (phenyl C), 128.5 (phenyl C), 128.0 (C6H6), 127.1 (phenyl C), 126.4 (phenyl C), 126.3 (phenyl C), 126.1 (phenyl C), 124.4 (phenyl C), 124.3 (phenyl C), 124.2 (phenyl C), 123.0 (ring C), 11.7 (CH3), −0.4 (Si(CH3)3) ppm. IR (KBr, cm−1): 2962 (s), 1596 (m), 1439 (m), 1383 (s), 1260 (s), 1091 (s), 1019 (s), 799 (s).
N), 148.1 (CPh), 147.2 (CPh), 146.8 (CPh), 143.2 (phenyl C), 140.0 (phenyl C), 135.3 (phenyl C), 133.8 (phenyl C), 132.7 (phenyl C), 130.4 (phenyl C), 129.5 (phenyl C), 128.9 (phenyl C), 128.5 (phenyl C), 128.0 (C6H6), 127.1 (phenyl C), 126.4 (phenyl C), 126.3 (phenyl C), 126.1 (phenyl C), 124.4 (phenyl C), 124.3 (phenyl C), 124.2 (phenyl C), 123.0 (ring C), 11.7 (CH3), −0.4 (Si(CH3)3) ppm. IR (KBr, cm−1): 2962 (s), 1596 (m), 1439 (m), 1383 (s), 1260 (s), 1091 (s), 1019 (s), 799 (s).
Method B. NMR scale. An n-hexane (10.0 μL, 2 M) solution of Me3SiCHN2 (0.02 mmol) was slowly added to a J. Young NMR tube charged with (η5-C5Me5)2Th(η2-C4Ph4) (2; 17 mg, 0.02 mmol) and C6D6 (0.5 mL). The color of the solution immediately changed from yellow to red, and resonances corresponding to 3 along with those of n-hexane were observed by 1H NMR spectroscopy (100% conversion in 10 min).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) C(C12H8))(C4Ph4)]·C6H12 (4·C6H12).
Method A. This compound was prepared as green crystals from the reaction of (η5-C5Me5)2Th(η2-C4Ph4) (2; 215 mg, 0.25 mmol) and 9-diazofluorene (48 mg, 0.25 mmol) in toluene (15 mL) and recrystallization from a cyclohexane solution by a similar procedure as in the synthesis of 3. Yield: 255 mg (90%) (found: C, 70.79; H, 6.30; N, 2.41. C67H70N2Th requires C, 70.88; H, 6.21; N, 2.47%). M.p.: 166–168 °C (decomp.). 1H NMR (C6D6): δ 8.19 (d, J = 7.8 Hz, 2H, aryl), 7.31 (t, J = 8.3 Hz, 2H, aryl), 7.24 (m, 4H, aryl), 7.11 (m, 6H, aryl), 7.03 (m, 4H, aryl), 6.91 (t, J = 6.1 Hz, 2H, aryl), 6.80 (m, 4H, aryl), 6.66 (t, J = 7.4 Hz, 1H, aryl), 6.43 (t, J = 7.3 Hz, 2H, aryl), 6.29 (t, J = 7.3 Hz, 1H, aryl), 2.42 (s, 15H, CH3), 1.71 (s, 15H, CH3), 1.40 (s, 12H, C6H12) ppm. 13C{1H} NMR (C6D6): δ 215.7 (ThCPh), 150.0 (CPh), 150.0 (CPh), 148.3 (CPh), 146.5 (aryl C), 143.0 (aryl C), 139.9 (aryl C), 138.8 (aryl C), 137.4 (aryl C), 135.5 (aryl C), 134.1 (aryl C), 133.9 (aryl C), 133.4 (aryl C), 132.5 (aryl C), 128.5 (aryl C), 127.6 (aryl C), 127.0 (aryl C), 126.9 (aryl C), 126.7 (aryl C), 126.6 (aryl C), 126.5 (aryl C), 126.3 (aryl C), 125.9 (aryl C), 125.6 (aryl C), 125.4 (aryl C), 125.2 (aryl C), 124.7 (aryl C), 123.3 (aryl C), 121.4 (aryl C), 120.3 (aryl C), 119.4 (ring C), 27.2 (C6H12), 12.8 (CH3), 11.4 (CH3) ppm. IR (KBr, cm−1): 2962 (m), 1584 (m), 1434 (s), 1384 (s), 1260 (s), 1094 (s), 1019 (s), 803 (s).
C(C12H8))(C4Ph4)]·C6H12 (4·C6H12).
Method A. This compound was prepared as green crystals from the reaction of (η5-C5Me5)2Th(η2-C4Ph4) (2; 215 mg, 0.25 mmol) and 9-diazofluorene (48 mg, 0.25 mmol) in toluene (15 mL) and recrystallization from a cyclohexane solution by a similar procedure as in the synthesis of 3. Yield: 255 mg (90%) (found: C, 70.79; H, 6.30; N, 2.41. C67H70N2Th requires C, 70.88; H, 6.21; N, 2.47%). M.p.: 166–168 °C (decomp.). 1H NMR (C6D6): δ 8.19 (d, J = 7.8 Hz, 2H, aryl), 7.31 (t, J = 8.3 Hz, 2H, aryl), 7.24 (m, 4H, aryl), 7.11 (m, 6H, aryl), 7.03 (m, 4H, aryl), 6.91 (t, J = 6.1 Hz, 2H, aryl), 6.80 (m, 4H, aryl), 6.66 (t, J = 7.4 Hz, 1H, aryl), 6.43 (t, J = 7.3 Hz, 2H, aryl), 6.29 (t, J = 7.3 Hz, 1H, aryl), 2.42 (s, 15H, CH3), 1.71 (s, 15H, CH3), 1.40 (s, 12H, C6H12) ppm. 13C{1H} NMR (C6D6): δ 215.7 (ThCPh), 150.0 (CPh), 150.0 (CPh), 148.3 (CPh), 146.5 (aryl C), 143.0 (aryl C), 139.9 (aryl C), 138.8 (aryl C), 137.4 (aryl C), 135.5 (aryl C), 134.1 (aryl C), 133.9 (aryl C), 133.4 (aryl C), 132.5 (aryl C), 128.5 (aryl C), 127.6 (aryl C), 127.0 (aryl C), 126.9 (aryl C), 126.7 (aryl C), 126.6 (aryl C), 126.5 (aryl C), 126.3 (aryl C), 125.9 (aryl C), 125.6 (aryl C), 125.4 (aryl C), 125.2 (aryl C), 124.7 (aryl C), 123.3 (aryl C), 121.4 (aryl C), 120.3 (aryl C), 119.4 (ring C), 27.2 (C6H12), 12.8 (CH3), 11.4 (CH3) ppm. IR (KBr, cm−1): 2962 (m), 1584 (m), 1434 (s), 1384 (s), 1260 (s), 1094 (s), 1019 (s), 803 (s).
Method B. NMR scale. To a J. Young NMR tube charged with (η5-C5Me5)2Th(η2-C4Ph4) (2; 17 mg, 0.02 mmol) and C6D6 (0.5 mL), 9-diazofluorene (3.8 mg, 0.02 mmol) was added. The color of the solution immediately changed from yellow to green, and the NMR resonances of 4 were observed by 1H NMR spectroscopy (100% conversion in 10 min).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N(p-tolyl))(C4Ph4)N(N
N(p-tolyl))(C4Ph4)N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N(p-tolyl))] (5).
Method A. This compound was prepared as red crystals from the reaction of (η5-C5Me5)2Th(η2-C4Ph4) (2; 215 mg, 0.25 mmol) and p-tolylN3 (67 mg, 0.50 mmol) in toluene (15 mL) and recrystallization from a THF solution by a similar procedure as in the synthesis of 3. Yield: 230 mg (82%) (found: C, 66.15; H, 5.82; N, 7.48. C62H64N6Th requires C, 66.18; H, 5.73; N, 7.47%). M.p.: 210–212 °C (decomp.). 1H NMR (C6D6): δ 7.68 (d, J = 8.4 Hz, 4H, phenyl), 7.34 (d, J = 7.0 Hz, 4H, phenyl), 7.19 (m, 8H, phenyl), 6.81 (t, J = 7.6 Hz, 4H, phenyl), 6.76 (m, 6H, phenyl), 6.58 (t, J = 7.3 Hz, 2H, phenyl), 2.24 (s, 6H, tolylCH3), 1.91 (s, 30H, CH3) ppm. 13C{1H} NMR (C6D6): δ 147.7 (CPh), 143.3 (CPh), 141.7 (phenyl C), 138.5 (phenyl C), 133.5 (phenyl C), 132.8 (phenyl C), 132.6 (phenyl C), 131.2 (phenyl C), 129.9 (phenyl C), 127.5 (phenyl C), 127.4 (phenyl C), 127.3 (phenyl C), 127.1 (phenyl C), 125.7 (phenyl C), 118.4 (ring C), 20.9 (tolylCH3), 12.9 (CH3) ppm. IR (KBr, cm−1): 2918 (s), 1605 (m), 1505 (s), 1442 (s), 1300 (s), 1258 (s), 1190 (s), 1090 (s), 1026 (s), 818 (s).
N(p-tolyl))] (5).
Method A. This compound was prepared as red crystals from the reaction of (η5-C5Me5)2Th(η2-C4Ph4) (2; 215 mg, 0.25 mmol) and p-tolylN3 (67 mg, 0.50 mmol) in toluene (15 mL) and recrystallization from a THF solution by a similar procedure as in the synthesis of 3. Yield: 230 mg (82%) (found: C, 66.15; H, 5.82; N, 7.48. C62H64N6Th requires C, 66.18; H, 5.73; N, 7.47%). M.p.: 210–212 °C (decomp.). 1H NMR (C6D6): δ 7.68 (d, J = 8.4 Hz, 4H, phenyl), 7.34 (d, J = 7.0 Hz, 4H, phenyl), 7.19 (m, 8H, phenyl), 6.81 (t, J = 7.6 Hz, 4H, phenyl), 6.76 (m, 6H, phenyl), 6.58 (t, J = 7.3 Hz, 2H, phenyl), 2.24 (s, 6H, tolylCH3), 1.91 (s, 30H, CH3) ppm. 13C{1H} NMR (C6D6): δ 147.7 (CPh), 143.3 (CPh), 141.7 (phenyl C), 138.5 (phenyl C), 133.5 (phenyl C), 132.8 (phenyl C), 132.6 (phenyl C), 131.2 (phenyl C), 129.9 (phenyl C), 127.5 (phenyl C), 127.4 (phenyl C), 127.3 (phenyl C), 127.1 (phenyl C), 125.7 (phenyl C), 118.4 (ring C), 20.9 (tolylCH3), 12.9 (CH3) ppm. IR (KBr, cm−1): 2918 (s), 1605 (m), 1505 (s), 1442 (s), 1300 (s), 1258 (s), 1190 (s), 1090 (s), 1026 (s), 818 (s).
Method B. NMR scale. A C6D6 (0.3 mL) solution of p-tolylN3 (5.2 mg, 0.04 mmol) was slowly added to a J. Young NMR tube charged with (η5-C5Me5)2Th(η2-C4Ph4) (2; 17 mg, 0.02 mmol) and C6D6 (0.2 mL). The color of the solution immediately changed from yellow to red, and resonances due to 5 were observed by 1H NMR spectroscopy (100% conversion in 10 min).
X-ray crystallography
Single-crystal X-ray diffraction measurements were carried out on a Bruker SMART CCD diffractometer at 113(2) K using graphite monochromated Mo Kα radiation (λ = 0.71073 Å). An empirical absorption correction was applied using the SADABS program.13 All structures were solved by direct methods and refined by full-matrix least squares on F2 using the SHELXL-97 program package.14 All the hydrogen atoms were geometrically fixed using the riding model. Disordered solvents in the voids of 5 were modeled or removed by using the SQUEEZE program.15 The crystal data and experimental data for 2–5 are summarized in Table 1. Selected bond lengths and angles are listed in Table 2.| Compound | 2 | 3·0.5C6H6 | 4·C6H12 | 5 |
|---|---|---|---|---|
| Formula | C48H50Th | C55H63N2SiTh | C67H70N2Th | C62H64N6Th |
| Fw | 858.92 | 1012.20 | 1135.29 | 1125.23 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Monoclinic |
| Space group | C2/c | P21/n |
P(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) ) |
C2/c |
| a (Å) | 13.662(1) | 14.231(3) | 11.359(3) | 48.165(10) |
| b (Å) | 16.932(1) | 22.998(5) | 11.964(3) | 10.309(2) |
| c (Å) | 17.245(1) | 14.519(3) | 20.965(5) | 26.366(6) |
| α (°) | 90 | 90 | 102.21(1) | 90 |
| β (°) | 103.46(1) | 91.54(1) | 100.72(1) | 118.01(1) |
| γ (°) | 90 | 90 | 91.28(1) | 90 |
| V (Å3) | 3879.6(5) | 4750.2(19) | 2730.4(12) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558(4) 558(4) |
| Z | 4 | 4 | 2 | 8 |
| D calc (g cm−3) | 1.471 | 1.415 | 1.381 | 1.293 |
| μ(Mo Kα)calc (cm−1) | 3.875 | 3.202 | 2.773 | 2.621 |
| Size (mm) | 0.35 × 0.27 × 0.21 | 0.30 × 0.20 × 0.20 | 0.35 × 0.30 × 0.30 | 0.30 × 0.20 × 0.20 |
| F(000) | 1712 | 2044 | 1152 | 4544 |
| 2θ range (°) | 3.90 to 55.14 | 3.96 to 55.10 | 3.50 to 55.24 | 3.50 to 55.00 |
| No. of reflns, collected | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 305 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258 258 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754 754 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230 |
| No. of obsd reflns | 4452 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 866 866 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 584 584 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 259 259 |
| No. of variables | 227 | 545 | 641 | 634 |
| Abscorr (Tmax, Tmin) | 0.50, 0.34 | 0.75, 0.60 | 0.75, 0.64 | 0.75, 0.63 |
| R | 0.018 | 0.033 | 0.031 | 0.036 |
| R w | 0.041 | 0.068 | 0.070 | 0.075 |
| R all | 0.042 | 0.073 | 0.072 | 0.080 |
| Gof | 1.07 | 0.99 | 1.04 | 1.00 |
| CCDC | 1033600 | 1033602 | 1033601 | 1033603 |
| Compound | C(Cp)–Thb | C(Cp)–Thc | Cp(cent)–Thb | Th–X | Cp(cent)–Th–Cp(cent) | X–Th–X/Y |
|---|---|---|---|---|---|---|
| a Cp = cyclopentadienyl ring. b Average value. c Range. d The angle of C(21)–Th(1)–N(2). e The angle of C(37)–Th(1)–N(2). f The angle of N(3)–Th(1)–N(6). | ||||||
| 2 | 2.814(2) | 2.784(2) to 2.840(2) | 2.543(2) | C(17) or C(17A) 2.465(2) | 144.5(1) | 74.1(1) |
| 3 | 2.859(4) | 2.826(3) to 2.924(4) | 2.592(4) | C(21) 2.545(3) | 138.9(1) | 69.4(1)d |
| N(1) 2.528(3) | ||||||
| N(2) 2.298(3) | ||||||
| 4 | 2.850(3) | 2.819(3) to 2.866(3) | 2.584(3) | C(37) 2.537(3) | 133.3(1) | 68.2(1)e |
| N(1) 2.568(2) | ||||||
| N(2) 2.304(2) | ||||||
| 5 | 2.872(4) | 2.808(3) to 2.938(4) | 2.592(3) | N(1) 2.537(3) N(3) 2.623(3) | 138.9(1) | 58.9(1)f |
| N(4) 2.565(3) N(6) 2.594(3) | ||||||
Computational methods
All calculations were carried out with the Gaussian 09 program (G09),16 employing the B3PW91 functional, plus a polarizable continuum model (PCM) and D317 (denoted as B3PW91-PCM+D3), with the standard 6-31G(d) basis set for C, H, N and Si elements and Stuttgart RLC ECP from the EMSL basis set exchange (https://bse.pnl.gov/bse/portal) for Th and U,18 to fully optimize the structures of reactants, complexes, transition states, intermediates, and products, and also to mimic the experimental toluene-solvent conditions (dielectric constant ε = 2.379). All stationary points were subsequently characterized by vibrational analyses, from which their respective zero-point (vibrational) energy (ZPE) were extracted and used in the relative energy determinations; in addition frequency calculations were also performed to ensure that the reactant, complex, intermediate, product and transition state structures resided at minima and 1st order saddle points, respectively, on their potential energy hyper surfaces.Results and discussion
Reduction of (η5-C5Me5)2ThCl2 (1) with an excess of KC8 in the presence of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh yielded the metallacyclopentadiene, (η5-C5Me5)2Th(η2-C4Ph4) (2) in 75% yield (Scheme 2). In contrast to the sterically more encumbered [η5-1,2,4-(Me3C)3C5H2]2Th fragment,9 no thorium metallacyclopropene was isolated regardless of the amount of PhC
CPh yielded the metallacyclopentadiene, (η5-C5Me5)2Th(η2-C4Ph4) (2) in 75% yield (Scheme 2). In contrast to the sterically more encumbered [η5-1,2,4-(Me3C)3C5H2]2Th fragment,9 no thorium metallacyclopropene was isolated regardless of the amount of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh employed. Complex 2 is air and moisture sensitive, but it can be obtained as yellow crystals from a benzene solution. Various spectroscopic techniques, elemental analysis and single crystal X-ray diffraction were employed to fully characterize complex 2. The 1H NMR spectrum of 2 shows narrow and well-resolved resonances in the range of 0–10 ppm, which is consistent with a diamagnetic molecule. In addition, the resonance in the 13C NMR spectrum at δ = 221.2 ppm is characteristic for the coordinated [η2-PhC
CPh employed. Complex 2 is air and moisture sensitive, but it can be obtained as yellow crystals from a benzene solution. Various spectroscopic techniques, elemental analysis and single crystal X-ray diffraction were employed to fully characterize complex 2. The 1H NMR spectrum of 2 shows narrow and well-resolved resonances in the range of 0–10 ppm, which is consistent with a diamagnetic molecule. In addition, the resonance in the 13C NMR spectrum at δ = 221.2 ppm is characteristic for the coordinated [η2-PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh–CPh
CPh–CPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh] group.
CPh] group.
The molecular structure of 2 is shown in Fig. 1. To the best of our knowledge, 2 represents the first structurally characterized thorium metallacyclopentadiene complex, and the crystal structure of the related uranium metallacycle, (η5-C5Me5)2U(η2-C4Ph4),4e is the only other reported actinide metallacyclopentadiene complex. The distance Th–C(17) or Th–C(17A) of 2.465(2) Å is longer than that (2.395(2) Å) found in metallacyclopropene [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2),9 but it is comparable to the other reported Th–C(sp2) σ-bonds (2.420(3)–2.654(14) Å)19 and slightly longer than the U–C distance in (η5-C5Me5)2U(η2-C4Ph4) (2.395 (2) Å).4e The angle (74.1(1)°) of C(17)–Th(1)–C(17A) is larger than that (32.6(1)°) found in metallacyclopropene [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2).9 Furthermore, the distance (1.362(3) Å) of C(17)–C(18) is shorter than that (1.516(4) Å) of C(18)–C(18A), consistent with a localized metallacyclopentadiene structure as previously shown for the uranium metallacyclopentadiene (η5-C5Me5)2U(η2-C4Ph4),4e in which the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C distances are 1.365(3) and 1.509(4) Å, respectively.4e The reduced steric strain of the metallacyclopentadiene moiety should be reflected in a different reactivity compared to that of [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2).9
C and C–C distances are 1.365(3) and 1.509(4) Å, respectively.4e The reduced steric strain of the metallacyclopentadiene moiety should be reflected in a different reactivity compared to that of [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2).9
In order to better understand the electronic structure of complex 2, we undertook computational studies at the DFT level of theory. In addition, we decided to compare the bonding in 2 to its uranium analogue (η5-C5Me5)2U(η2-C4Ph4). The DFT computations reproduce well the experimentally determined geometries of 2 and (η5-C5Me5)2U(η2-C4Ph4), in which the butadiene fragment is coordinated to the (η5-C5Me5)2An fragment by two An–C σ-bonds, as illustrated in Fig. 2. Furthermore, the natural bond orbital (NBO) analysis (Table 3) reveals that in complex 2, the strongly polarized Th–C σ-bonds (σ Th–C) are composed of a carbon sp2-hybrid orbital (89.8%; 29% s and 71% p) and a thorium hybrid orbital (10.2%; 20% 5f and 48% 6d and 4% 7p and 28% 7s). In contrast, the bonding in the uranium complex (η5-C5Me5)2U(η2-C4Ph4) is more covalent (17.4% U) and 5f orbitals also play a more important role. The percent contribution of 5f orbitals to U–C σ-bonds is substantially larger in the uranium complex (η5-C5Me5)2U(η2-C4Ph4) (37%) than that in the thorium complex 2 (20%). Furthermore, one C–C σ-bond (σ C–C) is composed of pure sp2-hybrid orbitals. Moreover, two bonding orbitals are found for the two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds: one is a σ-bond (σ C
C bonds: one is a σ-bond (σ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) with pure sp2-hybrid orbitals; the other bonding orbital is a π-bond (π C
C) with pure sp2-hybrid orbitals; the other bonding orbital is a π-bond (π C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) with pure p orbitals. Overall, these computations reveal that the An–C bonds in actinide metallacyclopentadienes are rather ionic, but also demonstrate that actinide 5f orbitals are indeed involved in the bonding between the metallocene and C4Ph4 fragments. This is consistent with previous conclusions that the 5f orbitals play an important role in the bonding of actinide complexes.19a,20
C) with pure p orbitals. Overall, these computations reveal that the An–C bonds in actinide metallacyclopentadienes are rather ionic, but also demonstrate that actinide 5f orbitals are indeed involved in the bonding between the metallocene and C4Ph4 fragments. This is consistent with previous conclusions that the 5f orbitals play an important role in the bonding of actinide complexes.19a,20
| 2 (Th) | (η5-C5Me5)2U(η2-C4Ph4) | ||
|---|---|---|---|
| σ An–C | %An | 10.2 | 17.4 |
| %s | 28 | 16 | |
| %p | 4 | 6 | |
| %d | 48 | 41 | |
| %f | 20 | 37 | |
| %C | 89.8 | 82.6 | |
| %s | 29 | 30 | |
| %p | 71 | 70 | |
| σ C–C | %s | 31 | 32 |
| %p | 69 | 68 | |
σ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
%s | 38 | 33 |
| %p | 62 | 67 | |
π C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
%p | 100 | 100 |
The next step was to probe the intrinsic reactivity of complex 2 and to compare these results to those of the thorium metallacyclopropene complex [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2).9 In contrast to the thorium metallacyclopropene,9 the reaction products of 2 with one equivalent of diazoalkanes Me3SiCHN2 or 9-diazofluorene yielded the six-membered hydrazido complexes (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSiMe3)(C4Ph4)] (3) and (η5-C5Me5)2Th[N(N
CHSiMe3)(C4Ph4)] (3) and (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C12H8))(C4Ph4)] (4) in quantitative conversions (Scheme 3). According to DFT computations the adduct COM is initially formed in the reaction of 2 with Me3SiCHN2 and the insertion of the coordinated Me3SiCHN2 proceeds via the transition state TS (Fig. 3). Interestingly, attempts to optimize a side-on bound (η2-N,N) adduct failed, instead the end-on bound adduct COM is always formed because of the steric hindrance. In the TS the two forming bond distances Th–N and C–N are 2.291 and 2.061 Å, respectively, which can be compared to those found in the product 3 of 2.308 and 1.376 Å, respectively. In the transition state the Th–N and C–N bonds are formed simultaneously, while the other nitrogen atom is coordinated to thorium ion. The conversion of COM to the product 3 is energetically very favorable by ΔG° = −37.4 kcal mol−1 and the activation barrier (ΔG‡) is 21.2 kcal mol−1, which is consistent with the rapid formation of 3 at ambient temperature.
C(C12H8))(C4Ph4)] (4) in quantitative conversions (Scheme 3). According to DFT computations the adduct COM is initially formed in the reaction of 2 with Me3SiCHN2 and the insertion of the coordinated Me3SiCHN2 proceeds via the transition state TS (Fig. 3). Interestingly, attempts to optimize a side-on bound (η2-N,N) adduct failed, instead the end-on bound adduct COM is always formed because of the steric hindrance. In the TS the two forming bond distances Th–N and C–N are 2.291 and 2.061 Å, respectively, which can be compared to those found in the product 3 of 2.308 and 1.376 Å, respectively. In the transition state the Th–N and C–N bonds are formed simultaneously, while the other nitrogen atom is coordinated to thorium ion. The conversion of COM to the product 3 is energetically very favorable by ΔG° = −37.4 kcal mol−1 and the activation barrier (ΔG‡) is 21.2 kcal mol−1, which is consistent with the rapid formation of 3 at ambient temperature.
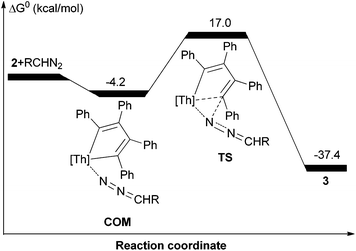 | ||
| Fig. 3 Free energy profile (kcal mol−1) for the reactions of 2 + Me3SiCHN2. [Th] = (η5-C5Me5)2Th. R = Me3Si. | ||
Furthermore, in contrast with the thorium metallacyclopropene [η5-1,2,4-(Me3C)3C5H2]2Th(η2-C2Ph2),9 complex 2 reacts with organic azides such as p-tolylN3 to the bis(triazenido) complex (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))(C4Ph4)N(N
N(p-tolyl))(C4Ph4)N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))] (5) (Scheme 3). Double insertion of p-tolylN3 into the Th–C σ-bonds appears to be more favorable in this case, since the bis(triazenido) moiety [N(N
N(p-tolyl))] (5) (Scheme 3). Double insertion of p-tolylN3 into the Th–C σ-bonds appears to be more favorable in this case, since the bis(triazenido) moiety [N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))(C4Ph4)N(N
N(p-tolyl))(C4Ph4)N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))]2− is formed irrespectively of the amount of p-tolylN3 employed.
N(p-tolyl))]2− is formed irrespectively of the amount of p-tolylN3 employed.
Complexes 3–5 are air and moisture sensitive and they could be characterized by various spectroscopic techniques, elemental analyses and single crystal X-ray diffraction analyses. The solid state molecular structures of (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSiMe3)(C4Ph4)] (3) and (η5-C5Me5)2Th[N(N
CHSiMe3)(C4Ph4)] (3) and (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C12H8))(C4Ph4)] (4) are shown in Fig. 4 and 5. The average Th–C(Cp) distances in 3 and 4 are virtually identical with 2.859(4) Å and 2.850(4) Å, respectively, whereas the angle Cp(cent)–Th–Cp(cent) in 3 with 138.9(1)° is slightly larger than that in 4 with 133.3(1)°. Furthermore, Th–C (C(21) for 3 and C(37) for 4) distances of 2.545(3) Å and 2.537(3) Å, respectively, are elongated compared to those found in 2 (2.465(2) Å). The N(1)–N(2) distances of 1.381(4) Å and 1.367(3) Å for 3 and 4, respectively, are comparable to that found in [η5-1,2,4-(Me3C)3C5H2][η5:σ-1,2-(Me3C)2-4-CMe2(CH2NN
C(C12H8))(C4Ph4)] (4) are shown in Fig. 4 and 5. The average Th–C(Cp) distances in 3 and 4 are virtually identical with 2.859(4) Å and 2.850(4) Å, respectively, whereas the angle Cp(cent)–Th–Cp(cent) in 3 with 138.9(1)° is slightly larger than that in 4 with 133.3(1)°. Furthermore, Th–C (C(21) for 3 and C(37) for 4) distances of 2.545(3) Å and 2.537(3) Å, respectively, are elongated compared to those found in 2 (2.465(2) Å). The N(1)–N(2) distances of 1.381(4) Å and 1.367(3) Å for 3 and 4, respectively, are comparable to that found in [η5-1,2,4-(Me3C)3C5H2][η5:σ-1,2-(Me3C)2-4-CMe2(CH2NN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSiMe3)C5H2]Th[NH(p-tolyl)] (1.366(8) Å).8 The Th–N(1) distances of 2.528(3) Å and 2.568(2) Å in 3 and 4, respectively, are relatively long and indicative of datively coordinated nitrogen atoms and in the same range as those found in [η5-1,2,4-(Me3C)3C5H2]2ThO(4-Me2NC5H4N) (2.587(5) Å)10 and [η5-1,2,4-(Me3C)3C5H2]2Th[(bipy)(SCPh2)] (2.564(1) Å).21 In contrast, the Th–N(2) distances of 2.298(3) Å and 2.304(2) Å for 3 and 4, respectively, are significantly shorter and can be compared to those found in [η5-1,2,4-(Me3C)3C5H2]2Th(NHp-tolyl)2 (2.279(3) and 2.286(3) Å),6 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(S)–S] (2.347(6) Å),8 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(NPh)–S] (2.328(3) Å),5 and [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)N
CHSiMe3)C5H2]Th[NH(p-tolyl)] (1.366(8) Å).8 The Th–N(1) distances of 2.528(3) Å and 2.568(2) Å in 3 and 4, respectively, are relatively long and indicative of datively coordinated nitrogen atoms and in the same range as those found in [η5-1,2,4-(Me3C)3C5H2]2ThO(4-Me2NC5H4N) (2.587(5) Å)10 and [η5-1,2,4-(Me3C)3C5H2]2Th[(bipy)(SCPh2)] (2.564(1) Å).21 In contrast, the Th–N(2) distances of 2.298(3) Å and 2.304(2) Å for 3 and 4, respectively, are significantly shorter and can be compared to those found in [η5-1,2,4-(Me3C)3C5H2]2Th(NHp-tolyl)2 (2.279(3) and 2.286(3) Å),6 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(S)–S] (2.347(6) Å),8 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(NPh)–S] (2.328(3) Å),5 and [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NN(p-tolyl)] (2.366(3) and 2.354(3) Å),8 [η5-1,2,4-(Me3C)3C5H2]2Th(bipy) (2.325(5) and 2.363(4) Å),22 and [η5-1,3-(Me3C)2C5H3]2Th(bipy) (2.326(7) and 2.325(7) Å).23
NN(p-tolyl)] (2.366(3) and 2.354(3) Å),8 [η5-1,2,4-(Me3C)3C5H2]2Th(bipy) (2.325(5) and 2.363(4) Å),22 and [η5-1,3-(Me3C)2C5H3]2Th(bipy) (2.326(7) and 2.325(7) Å).23
Fig. 6 depicts the molecular structure of (η5-C5Me5)2Th[N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))(C4Ph4)N(N
N(p-tolyl))(C4Ph4)N(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(p-tolyl))] (5). The N–N distances of 1.335(4) Å for N(1)–N(2), 1.310(4) Å for N(2)–N(3), 1.318(4) Å for N(4)–N(5), and 1.309(4) Å for N(5)–N(6), are nearly identical and consistent with a delocalization of the negative charge within the triazenyl fragments N(1)–N(2)–N(3) and N(4)–N(5)–N(6). Hence the Th–N distances of 2.537(3) Å for Th–N(1), 2.623(3) Å for Th–N(3), 2.565(3) Å for Th–N(4), and 2.594(3) Å for N(6)–Th(1) are much longer than those found in 3 (2.298(3) Å), 4 (2.304(2) Å), [η5-1,2,4-(Me3C)3C5H2]2Th(NHp-tolyl)2 (2.279(3) and 2.286(3) Å),6 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(S)–S] (2.347(6) Å),5 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(NPh)–S] (2.328(3) Å),5 and [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)N
N(p-tolyl))] (5). The N–N distances of 1.335(4) Å for N(1)–N(2), 1.310(4) Å for N(2)–N(3), 1.318(4) Å for N(4)–N(5), and 1.309(4) Å for N(5)–N(6), are nearly identical and consistent with a delocalization of the negative charge within the triazenyl fragments N(1)–N(2)–N(3) and N(4)–N(5)–N(6). Hence the Th–N distances of 2.537(3) Å for Th–N(1), 2.623(3) Å for Th–N(3), 2.565(3) Å for Th–N(4), and 2.594(3) Å for N(6)–Th(1) are much longer than those found in 3 (2.298(3) Å), 4 (2.304(2) Å), [η5-1,2,4-(Me3C)3C5H2]2Th(NHp-tolyl)2 (2.279(3) and 2.286(3) Å),6 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(S)–S] (2.347(6) Å),5 [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)C(NPh)–S] (2.328(3) Å),5 and [η5-1,2,4-(Me3C)3C5H2]2Th[N(p-tolyl)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NN(p-tolyl)] (2.366(3) and 2.354(3) Å),8 [η5-1,2,4-(Me3C)3C5H2]2Th(bipy) (2.325(5) and 2.363(4) Å),22 and [η5-1,3-(Me3C)2C5H3]2Th(bipy) (2.326(7) and 2.325(7) Å).23
NN(p-tolyl)] (2.366(3) and 2.354(3) Å),8 [η5-1,2,4-(Me3C)3C5H2]2Th(bipy) (2.325(5) and 2.363(4) Å),22 and [η5-1,3-(Me3C)2C5H3]2Th(bipy) (2.326(7) and 2.325(7) Å).23
Conclusions
In conclusion, the first thorium metallacyclopentadiene complex, (η5-C5Me5)2Th(η2-C4Ph4) (2), was comprehensively studied. Similar to thorium metallacyclopropene,9 density functional theory (DFT) studies reveals that 5f orbitals contribute to the Th–C σ-bonds of the Th–(η2-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) moiety, and that the σ-bonds between the [η5-1,2,4-(Me3C)3C5H2]2Th2+ and the [C4Ph4]2− fragments are very polarized, which makes the insertion of unsaturated substrates favorable. However, when the steric strain of the metallacycle is reduced, the reaction chemistry changes, as illustrated by their reaction with organic azides and diazoalkanes. While the thorium metallacyclopropene yields rearranged products,9 the thorium metallacyclopentadiene shows mono- and double insertion of diazoalkanes and organic azides into the Th–C bond to yield hydrazido and bis(triazenido) ligands, respectively. Further studies on the intrinsic reactivity of actinide metallacycles are in progress and will be reported in due course.
C) moiety, and that the σ-bonds between the [η5-1,2,4-(Me3C)3C5H2]2Th2+ and the [C4Ph4]2− fragments are very polarized, which makes the insertion of unsaturated substrates favorable. However, when the steric strain of the metallacycle is reduced, the reaction chemistry changes, as illustrated by their reaction with organic azides and diazoalkanes. While the thorium metallacyclopropene yields rearranged products,9 the thorium metallacyclopentadiene shows mono- and double insertion of diazoalkanes and organic azides into the Th–C bond to yield hydrazido and bis(triazenido) ligands, respectively. Further studies on the intrinsic reactivity of actinide metallacycles are in progress and will be reported in due course.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21472013, 21172022, 21272026, 21373030), Beijing Municipal Commission of Education, and the Deutsche Forschungsgemeinschaft (DFG) through the Emmy-Noether program (WA 2513/2).Notes and references
- For selected reviews, see: (a) J. P. Collman and L. P. Hegedus, Principles and Applications of Organotransition Metal Chemistry, University Science Books, Mill Valley, CA, 1980 Search PubMed; (b) S. L. Buchwald and R. B. Nielsen, Chem. Rev., 1988, 88, 1047–1058 CrossRef CAS; (c) U. Rosenthal, V. V. Burlakov, P. Arndt, W. Baumann and A. Spannenberg, Organometallics, 2003, 22, 884–900 CrossRef CAS; (d) U. Rosenthal, V. V. Burlakov, P. Arndt, W. Baumann, A. Spannenberg and V. B. Shur, Eur. J. Inorg. Chem., 2004, 4739–4749 CrossRef CAS; (e) U. Rosenthal, Angew. Chem., Int. Ed., 2004, 43, 3882–3887 CrossRef CAS PubMed; (f) U. Rosenthal, V. V. Burlakov, P. Arndt, W. Baumann and A. Spannenberg, Organometallics, 2005, 24, 456–471 CrossRef CAS; (g) U. Rosenthal, V. V. Burlakov, M. A. Bach and T. Beweries, Chem. Soc. Rev., 2007, 36, 719–728 RSC; (h) N. Suzuki and D. Hashizume, Coord. Chem. Rev., 2010, 254, 1307–1326 CrossRef CAS PubMed; (i) H. S. La Pierre and K. Meyer, Prog. Inorg. Chem., 2014, 58, 303–415 CrossRef CAS; (j) K. D. J. Parker and M. D. Fryzuk, Organometallics, 2014 DOI:10.1021/om5010385.
- For selected recent papers on group 4 metallacyclopropenes and metallacyclopentadienes, see: (a) T. Beweries, C. Fischer, S. Peitz, V. V. Burlakov, P. Arndt, W. Baumann, A. Spannenberg, D. Heller and U. Rosenthal, J. Am. Chem. Soc., 2009, 131, 4463–4469 CrossRef CAS PubMed; (b) K. Kaleta, M. Ruhmann, O. Theilmann, T. Beweries, S. Roy, P. Arndt, A. Villinger, E. D. Jemmis, A. Schulz and U. Rosenthal, J. Am. Chem. Soc., 2011, 133, 5463–5473 CrossRef CAS PubMed; (c) M. Haehnel, M. Ruhmann, O. Theilmann, S. Roy, T. Beweries, P. Arndt, A. Spannenberg, A. Villinger, E. D. Jemmis, A. Schulz and U. Rosenthal, J. Am. Chem. Soc., 2012, 134, 15979–15991 CrossRef CAS PubMed; (d) M. Haehnel, S. Hansen, K. Schubert, P. Arndt, A. Spannenberg, H. Jiao and U. Rosenthal, J. Am. Chem. Soc., 2013, 135, 17556–17565 CrossRef CAS PubMed; (e) S. K. Podiyanachari, G. Kehr, C. Mück-Lichtenfeld, C. Daniliuc and G. Erker, J. Am. Chem. Soc., 2013, 135, 17444–17456 CrossRef PubMed; (f) L. Becker, P. Arndt, H. Jiao, A. Spannenberg and U. Rosenthal, Angew. Chem., Int. Ed., 2013, 52, 11396–11400 CrossRef CAS PubMed; (g) Y. Mizukami, H. Li, K. Nakajima, Z. Song and T. Takahashi, Angew. Chem., Int. Ed., 2013, 52, 11396–11400 CrossRef PubMed; (h) Ò. Àrias, A. R. Petrov, T. Banenberg, K. Altenburger, P. Arndt, P. G. Jones, U. Rosenthal and M. Tamm, Organometallics, 2014, 33, 1774–1786 CrossRef; (i) D. J. Mindiola, L. A. Watson, K. Meyer and G. L. Hillhouse, Organometallics, 2014, 33, 2760–2769 CrossRef CAS PubMed.
- For selected reviews, see: (a) E.-I. Negishi and T. Takahashi, Acc. Chem. Res., 1994, 27, 124–130 CrossRef CAS; (b) T. Takahashi and Y. Li, Zirconacyclopentadienes in Organic Synthesis, in Titanium and Zirconium in Organic Synthesis, Wiley-VCH, Weinheim, Germany, 2002, pp. 50–85 Search PubMed; (c) J. A. Varela and C. Saá, Chem. Rev., 2003, 103, 3787–3802 CrossRef CAS PubMed; (d) H. Braunschweig and T. Kupfer, Chem. Commun., 2011, 47, 10903–10914 RSC; (e) S. Roy, U. Rosenthal and E. D. Jemmis, Acc. Chem. Res., 2014, 47, 2917–2930 CrossRef CAS PubMed.
- For selected actinide metallacyclopropenes and metallacyclopentadienes, see: (a) P. J. Fagan, J. M. Manriquez, T. J. Marks, C. S. Day, S. H. Vollmer and V. W. Day, Organometallics, 1982, 1, 170–180 CrossRef CAS; (b) P. J. Fagan, J. M. Manriquez, E. A. Maatta, A. M. Seyam and T. J. Marks, J. Am. Chem. Soc., 1981, 103, 6650–6667 CrossRef CAS; (c) J. M. Manriquez, P. J. Fagan, T. J. Marks, S. H. Vollmer, C. S. Day and V. W. Day, J. Am. Chem. Soc., 1979, 101, 5075–5078 CrossRef CAS; (d) J. M. Manriquez, P. J. Fagan and T. J. Marks, J. Am. Chem. Soc., 1978, 100, 3939–3941 CrossRef CAS; (e) W. J. Evans, S. A. Kozimor and J. W. Ziller, Chem. Commun., 2005, 4681–4683 RSC; (f) L. Andrews, G. P. Kushto and C. J. Marsden, Chem. – Eur. J., 2006, 12, 8324–8335 CrossRef CAS PubMed; (g) M. Foyentin, G. Folcher and M. Ephritikhine, J. Chem. Soc., Chem. Commun., 1987, 494–495 RSC.
- W. Ren, G. Zi, D.-C. Fang and M. D. Walter, J. Am. Chem. Soc., 2011, 133, 13183–13196 CrossRef CAS PubMed.
- W. Ren, G. Zi, D.-C. Fang and M. D. Walter, Chem. – Eur. J., 2011, 17, 12669–12682 CrossRef CAS PubMed.
- W. Ren, E. Zhou, B. Fang, G. Zi, D.-C. Fang and M. D. Walter, Chem. Sci., 2014, 5, 3165–3172 RSC.
- W. Ren, E. Zhou, B. Fang, G. Hou, G. Zi, D.-C. Fang and M. D. Walter, Angew. Chem., Int. Ed., 2014, 53, 11310–11314 CrossRef CAS PubMed.
- B. Fang, W. Ren, G. Hou, G. Zi, D.-C. Fang, L. Maron and M. D. Walter, J. Am. Chem. Soc., 2014, 136, 17249–17261 CrossRef CAS PubMed.
- D. Sutton, Chem. Rev., 1993, 93, 995–1022 CrossRef CAS.
- M. A. Schwindt, T. Lejon and L. S. Hegedus, Organometallics, 1990, 9, 2814–2819 CrossRef CAS.
- B. Fiedler, D. Weiß and R. Beckert, Liebigs Ann./Recl., 1997, 613–615 CrossRef CAS.
- G. M. Sheldrick, SADABS, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Göttingen, Germany, 1996 Search PubMed.
- (a) G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structure from Diffraction Data, University of Göttingen, Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- SQUEEZE: P. V. D. Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194–201 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09 (Revision A.02), Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, Mol. Phys., 1991, 74, 1245–1263 CrossRef.
- (a) L. A. Seaman, E. A. Pedrick, T. Tsuchiya, G. Wu, E. Jakubikova and T. W. Hayton, Angew. Chem., Int. Ed., 2013, 52, 10589–10592 CrossRef CAS PubMed; (b) I. Korobkov, B. Vidjayacoumar, S. I. Gorelsky, P. Billone and S. Gambarotta, Organometallics, 2010, 29, 692–702 CrossRef CAS; (c) I. Korobkov, A. Arunachalampillai and S. Gambarotta, Organometallics, 2004, 23, 6248–6252 CrossRef CAS.
- For selected papers about the bonding of organoactinide complexes, see: (a) T. Cantat, C. R. Graves, K. C. Jantunen, C. J. Burns, B. L. Scott, E. J. Schelter, D. E. Morris, P. J. Hay and J. L. Kiplinger, J. Am. Chem. Soc., 2008, 130, 17537–17551 CrossRef CAS PubMed; (b) N. Barros, D. Maynau, L. Maron, O. Eisenstein, G. Zi and R. A. Andersen, Organometallics, 2007, 26, 5059–5065 CrossRef CAS; (c) A. Yahia and L. Maron, Organometallics, 2009, 28, 672–679 CrossRef CAS; (d) W. Ren, X. Deng, G. Zi and D.-C. Fang, Dalton Trans., 2011, 40, 9662–9664 RSC; (e) E. Ciliberto, S. D. Bella, A. Gulino, I. Fragala, J. L. Petersen and T. J. Marks, Organometallics, 1992, 11, 1727–1737 CrossRef CAS; (f) K. Tatsumi and A. Nakamura, J. Am. Chem. Soc., 1987, 109, 3195–3206 CrossRef CAS; (g) J. R. Walensky, R. L. Martin, J. W. Ziller and W. J. Evans, Inorg. Chem., 2010, 49, 10007–10012 CrossRef CAS PubMed; (h) B. M. Gardner, P. A. Cleaves, C. E. Kefalidis, J. Fang, L. Maron, W. Lewis, A. J. Blakea and S. T. Liddle, Chem. Sci., 2014, 5, 2489–2497 RSC.
- W. Ren, W. W. Lukens, G. Zi, L. Maron and M. D. Walter, Chem. Sci., 2013, 4, 1168–1174 RSC.
- W. Ren, G. Zi and M. D. Walter, Organometallics, 2012, 31, 672–679 CrossRef CAS.
- W. Ren, H. Song, G. Zi and M. D. Walter, Dalton Trans., 2012, 41, 5965–5973 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Cartesian coordinates of all stationary points optimized at the B3PW91-PCM+D3 level. CCDC 1033600–1033603. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt00838g |
| This journal is © The Royal Society of Chemistry 2015 |