Open Access Article
Open Access ArticleA one-dimensional coordination polymer based on Cu3-oximato metallacrowns bridged by benzene-1,4-dicarboxylato ligands: structure and magnetic properties†
Lilia
Croitor
*a,
Eduard B.
Coropceanu
b,
Oleg
Petuhov
b,
Karl W.
Krämer
c,
Svetlana G.
Baca
a,
Shi-Xia
Liu
c,
Silvio
Decurtins
c and
Marina S.
Fonari
a
aInstitute of Applied Physics, Academy of Sciences of R. Moldova, Academy str., 5 MD2028, Chisinau, Moldova. E-mail: croitor.lilia@gmail.com; Fax: +373 22 725887; Tel: +373 22 738154
bInstitute of Chemistry, Academy of Sciences of R. Moldova, Academy str., 3 MD2028, Chisinau, Moldova
cDepartment of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, 3012 Bern, Switzerland
First published on 20th March 2015
Abstract
A one-dimensional linear coordination polymer {[Cu3(μ3-OH)(2-pyao)3(bdc)]·6(H2O)}n (1) composed of trinuclear [Cu3(μ3-OH)(2-pyao)3]2+ metallacrown cores bridged by bis-carboxylato linkers has been obtained by treatment of copper(II) fluoride with pyridine-2-aldoxime (2-pyaoH) ligand and benzene-1,4-dicarboxylic acid (H2bdc). Magnetic susceptibility measurements show strong antiferromagnetic interactions between Cu(II) centers within the trinuclear metallacrown core with J = −430 cm−1.
Introduction
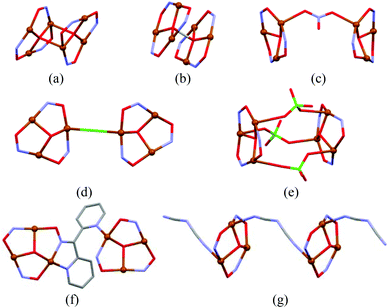
Metallacrowns (MCs) are a family of macrocyclic inorganic complexes with structural and functional similarity to crown ethers,1 that are composed of the –[M–N–O]n– repeating unit. The interest in the synthesis of copper(II) MC complexes and in the study of their properties is in the current focus due to their involvement in several catalytic processes in living organisms.2 For example, multicopper blue oxidases contain a triangular unit of copper atoms.3 In addition, cyclic trinuclear Cu(II) complexes can be regarded as spin-frustrated systems leading to unusual electronic properties3e,4 and therefore offer the opportunity to test magnetic exchange models which is a very topical issue in the field of molecular magnetism.4 In recent years, Cu3-oximato clusters derived from pyridine-2-oximes, (Py)C(R)NOH,5 have yielded a surprisingly rich chemistry: copper(II) 9-MC-3 have been reported for R = H,6 CN,7 Ph8 or Py.8a,9 Furthermore, several reports have appeared aiming to increase the nuclearity of magnetic clusters or to link small magnetic clusters through the self-assembly process towards the formation of multidimensional networks.10 These cores can further be extended to higher dimensional networks by other organic or inorganic linkers.10b–d The connection of Cu3 units in larger aggregates, e.g. their dimerization, has been achieved in a few cases by a μ4-oxo bridge (Scheme 1a),11 H-bonds between μ3-O/μ3-OH-centered triangles (Scheme 1b),12 and NO3−, Cl−, and ClO4− anions (Scheme 1c–e),13,14 as well as via additional donor arms or large flexible linkers between oximato groups (Scheme 1f).8a,12b,13 Notably, only a few examples of coordination polymers built up from the Cu3 coordination cores have been reported so far, namely 1D catena-((μ3-hydroxo)-tris(μ2-N-oxy-1-phenyl-1-(pyridin-2-yl)methanimine)-(μ2-dicyanamide)-(acetato)-tri-copper(II)),8b 1D catena-(bis(μ3-hydroxo)-bis(μ3-N-oxy-1-(pyrazin-2-yl)ethanimine)-tetrakis(μ2-N-oxy-1-(pyrazin-2-yl)ethanimine)-bis(methanol)-bis(nitrato-O)-hexa-copper dinitrate), and 3D catena-(bis(μ3-N-oxy-1-(pyrazin-2-yl)ethanimine)-(μ3-oxo)-di-copper bis(tetrafluoro-borate)).13 In the first one, the linkage of triangular Cu3 cores occurs through the “innocent” μ2-dicyanamide, N(CN)2− ligand, while in two other structures the extension occurs through pyrazyloximato ligands.Dicarboxylic acids, aromatic and aliphatic ones, demonstrate their excellent bridging functions in the metal–organic networks.15 Recently, we have reported some examples of 1D and 2D coordination polymers derived from the Zn/Cd oxime-chemistry, where the [M-pyridine-n-oxime] (n = 2, 4) mononuclear or binuclear units are combined into 1D and 2D polymeric networks via bridging benzene-1,4-dicarboxylato, malonato, succinato or adipato linkers.16 In continuing this research we report here the preparation, structural characterization and magnetic study of a novel 1D coordination polymer, {[Cu3(μ3-OH)(2-pyao)3(bdc)]·6(H2O)}n (1) (2-pyaoH = pyridine-2-aldoxime, H2bdc = benzene-1,4-dicarboxylic acid), composed of trinuclear [Cu3(μ3-OH)(2-pyao)3]2+ cations bridged by bdc2− linkers (Scheme 2) into chains.
Experimental
Materials and general methods
All reagents and solvents were obtained from commercial sources and were used without further purification. Elemental analysis was performed on an Elementar Analysensysteme GmbH Vario El III elemental analyzer. The IR spectrum was recorded by the ATR-IR spectroscopic technique on a FT IR Spectrum-100 Perkin Elmer spectrometer in the range of 650–4000 cm−1. The UV-Vis absorption spectrum was recorded on a UV-Vis Lambda 25 Perkin Elmer spectrometer. The thermogravimetric analysis (TGA) was carried out with a Derivatograph Q-1500 thermal analyzer in an air flow at a heating rate of 5 °C min−1 in the temperature range of 25–1000 °C. The nitrogen adsorption–desorption isotherm has been measured using an Autosorb-1MP device. Magnetic susceptibility data were recorded using a Quantum design MPMS-5XL SQUID magnetometer in the temperature range 1.9–280 K and at a field of 1 kG. Experimental data were corrected for sample holder and diamagnetic contributions (−0.45 × molecular weight × 10−6 cm3 mol−1).Synthesis of {[Cu3(μ3-OH)(2-pyao)3(bdc)]·6(H2O)}n (1)
CuF2 (0.01 g, 0.1 mmol) and 2-pyaoH (0.024 g, 0.2 mmol) were dissolved in a mixture of H2O–CH3OH–dmf (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40 ml). The solution was heated and stirred for 10 min, and then H2bdc (0.017g, 0.1 mmol) was added. The resulting solution was heated for 10 min, filtered off, and then slowly cooled to room temperature giving blue crystals. The crystals were washed with water and air-dried. They are soluble in alcohols, dmf, and partially in water. Yield 73%. Calc. for C52H40Cu6N12O22: C 39.88; H 2.57; N 10.74. Found C 39.31; H 2.14; N 10.48%. IR (cm−1): 3375 (m), 3097 (w), 3037 (w), 1648 (w), 1605 (s), 1562 (s), 1537 (s), 1474 (s), 1442 (m), 1370 (s), 1346 (m), 1301 (w), 1262 (w), 1218 (m), 1117 (vs), 1095 (vs), 1054 (m), 1041 (sh), 886 (m), 810 (m), 774 (sh), 753 (m), 685 (m).
1, 40 ml). The solution was heated and stirred for 10 min, and then H2bdc (0.017g, 0.1 mmol) was added. The resulting solution was heated for 10 min, filtered off, and then slowly cooled to room temperature giving blue crystals. The crystals were washed with water and air-dried. They are soluble in alcohols, dmf, and partially in water. Yield 73%. Calc. for C52H40Cu6N12O22: C 39.88; H 2.57; N 10.74. Found C 39.31; H 2.14; N 10.48%. IR (cm−1): 3375 (m), 3097 (w), 3037 (w), 1648 (w), 1605 (s), 1562 (s), 1537 (s), 1474 (s), 1442 (m), 1370 (s), 1346 (m), 1301 (w), 1262 (w), 1218 (m), 1117 (vs), 1095 (vs), 1054 (m), 1041 (sh), 886 (m), 810 (m), 774 (sh), 753 (m), 685 (m).
X-ray crystallography
A blue block-shaped crystal with dimensions 0.18 × 0.16 × 0.05 mm was used for structural analysis at 293(2) K on a Xcalibur “Oxford Diffraction” diffractometer equipped with a CCD area detector and a graphite monochromator utilizing Mo Kα radiation. Final unit cell dimensions were obtained and refined on an entire data set. All calculations to solve the structure and to refine the proposed model were carried out with the programs SHELXS97 and SHELXL97.17 The structure was solved by direct methods and refined by full-matrix least-squares methods on F2 by using the SHELXL97 program package. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms attached to carbon atoms were placed in geometrically idealized positions and refined by using a riding model. For the disordered solvent water molecules, hydrogen atoms were not localized. The X-ray data and the details of the refinement for 1 are summarized in Table 1. Selected geometric parameters for 1 are given in Table 2. The figures were produced using the Mercury program.18 The solvent accessible voids (SAVs) were calculated using PLATON.19 CCDC 1047595 contains the supplementary crystallographic data for 1.| Empirical formula | C52H40Cu6N12O22 |
| Formula weight | 1566.20 |
| Crystal system | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Z | 2 |
| a (Å) | 9.9884(11) |
| b (Å) | 17.0223(11) |
| c (Å) | 19.465(2) |
| α (°) | 75.950(8) |
| β (°) | 84.262(9) |
| γ (°) | 80.032(7) |
| V (Å3) | 3156.3(5) |
| D c (g cm–3) | 1.648 |
| μ (mm−1) | 2.069 |
| F(000) | 1572 |
| Crystal size (mm) | 0.18 × 0.16 × 0.05 |
| Reflections collected/unique | 17882/11335 [R(int) = 0.0504] |
| Reflections with [I > 2σ(I)] | 6146 |
| Data/restraints/parameters | 11335/0/865 |
| GOF on F2 | 1.100 |
| R 1, wR2 [I > 2σ(I)] | 0.0694, 0.0931 |
| R 1, wR2 (all data) | 0.1405, 0.1106 |
| Cu(1)–O(4) | 1.962(5) | Cu(4)–O(2) | 1.946(4) |
| Cu(1)–O(1) | 1.967(4) | Cu(4)–N(7) | 1.976(6) |
| Cu(1)–N(2) | 1.977(6) | Cu(4)–N(8) | 1.985(6) |
| Cu(1)–N(1) | 2.008(5) | Cu(4)–O(7) | 1.983(5) |
| Cu(1)–O(10) | 2.252(4) | Cu(4)–O(15) | 2.267(4) |
| Cu(2)–O(1) | 1.940(4) | Cu(5)–O(8) | 1.961(4) |
| Cu(2)–O(5) | 1.968(5) | Cu(5)–O(2) | 1.962(5) |
| Cu(2)–N(4) | 1.974(6) | Cu(5)–N(10) | 1.996(6) |
| Cu(2)–N(3) | 1.985(6) | Cu(5)–N(9) | 1.990(5) |
| Cu(2)–O(14) | 2.306(4) | Cu(5)–O(12) | 2.242(4) |
| Cu(3)–O(3) | 1.964(4) | Cu(6)–O(6) | 1.970(4) |
| Cu(3)–O(1) | 1.971(4) | Cu(6)–O(2) | 1.965(4) |
| Cu(3)–N(5) | 1.988(5) | Cu(6)–N(11) | 1.974(6) |
| Cu(3)–N(6) | 2.006(6) | Cu(6)–N(12) | 2.008(5) |
| Cu(3)–O(9) | 2.245(4) | Cu(6)–O(11) | 2.238(4) |
| O(4)–Cu(1)–O(1) | 93.46(18) | O(2)–Cu(4)–N(7) | 88.3(2) |
| O(4)–Cu(1)–N(2) | 170.30(19) | O(2)–Cu(4)–N(8) | 168.7(2) |
| O(1)–Cu(1)–N(2) | 88.6(2) | N(7)–Cu(4)–N(8) | 81.7(2) |
| O(4)–Cu(1)–N(1) | 94.6(2) | O(2)–Cu(4)–O(7) | 94.37(19) |
| O(1)–Cu(1)–N(1) | 164.3(2) | N(7)–Cu(4)–O(7) | 159.4(2) |
| N(2)–Cu(1)–N(1) | 81.2(2) | N(8)–Cu(4)–O(7) | 93.2(2) |
| O(4)–Cu(1)–O(10) | 89.09(19) | O(2)–Cu(4)–O(15) | 94.34(16) |
| O(1)–Cu(1)–O(10) | 89.65(16) | N(7)–Cu(4)–O(15) | 105.3(2) |
| N(2)–Cu(1)–O(10) | 100.41(19) | N(8)–Cu(4)–O(15) | 93.5(2) |
| N(1)–Cu(1)–O(10) | 103.92(19) | O(7)–Cu(4)–O(15) | 94.83(19) |
| O(1)–Cu(2)–O(5) | 94.77(19) | O(8)–Cu(5)–O(2) | 92.64(18) |
| O(1)–Cu(2)–N(4) | 168.8(2) | O(8)–Cu(5)–N(10) | 95.2(2) |
| O(5)–Cu(2)–N(4) | 93.3(2) | O(2)–Cu(5)–N(10) | 163.65(19) |
| O(1)–Cu(2)–N(3) | 89.1(2) | O(8)–Cu(5)–N(9) | 173.4(2) |
| O(5)–Cu(2)–N(3) | 159.4(2) | O(2)–Cu(5)–N(9) | 89.6(2) |
| N(4)–Cu(2)–N(3) | 80.6(2) | N(10)–Cu(5)–N(9) | 81.2(3) |
| O(1)–Cu(2)–O(14) | 91.44(16) | O(8)–Cu(5)–O(12) | 98.01(18) |
| O(5)–Cu(2)–O(14) | 92.08(18) | O(2)–Cu(5)–O(12) | 97.33(18) |
| N(4)–Cu(2)–O(14) | 95.93(19) | N(10)–Cu(5)–O(12) | 95.8(2) |
| N(3)–Cu(2)–O(14) | 108.0(2) | N(9)–Cu(5)–O(12) | 87.84(19) |
| O(3)–Cu(3)–O(1) | 93.19(17) | O(6)–Cu(6)–O(2) | 93.25(17) |
| O(3)–Cu(3)–N(5) | 176.7(2) | O(6)–Cu(6)–N(11) | 171.0(2) |
| O(1)–Cu(3)–N(5) | 88.6(2) | O(2)–Cu(6)–N(11) | 88.0(2) |
| O(3)–Cu(3)–N(6) | 96.1(2) | O(6)–Cu(6)–N(12) | 95.5(2) |
| O(1)–Cu(3)–N(6) | 163.24(19) | O(2)–Cu(6)–N(12) | 162.0(2) |
| N(5)–Cu(3)–N(6) | 81.5(2) | N(11)–Cu(6)–N(12) | 81.0(3) |
| O(3)–Cu(3)–O(9) | 92.27(17) | O(6)–Cu(6)–O(11) | 87.91(18) |
| O(1)–Cu(3)–O(9) | 99.78(18) | O(2)–Cu(6)–O(11) | 92.57(16) |
| N(5)–Cu(3)–O(9) | 90.1(2) | N(11)–Cu(6)–O(11) | 101.0(2) |
| N(6)–Cu(3)–O(9) | 93.8(2) | N(12)–Cu(6)–O(11) | 103.42(19) |
Results and discussion
General
The crystals of compound 1 were obtained by solvent cocrystallization of components in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of water–methanol–dmf solution. The dark blue crystals with an irregular shape are air-stable (Fig. 1S in ESI†). The optical absorption spectrum recorded in methanol solution reveals two bands (Fig. 2S†). The strongest band appears at λmax = 240 nm, which is predominantly due to an intraligand charge transfer (ILCT), and another one at λmax = 332 nm is due to a ligand to ligand charge transfer (LLCT). The IR spectrum of compound 1 shown in Fig. 3S† confirms the presence of the organic ligands through the typical vibrations of benzene/pyridine aromatic rings, oxime and carboxylic groups.8c A broad band centered at 3375 cm−1 is due to the presence of water molecules. Bands characteristic for the 2-pyao ligand are at 1605 cm−1 (s, pyridyl C
1 molar ratio of water–methanol–dmf solution. The dark blue crystals with an irregular shape are air-stable (Fig. 1S in ESI†). The optical absorption spectrum recorded in methanol solution reveals two bands (Fig. 2S†). The strongest band appears at λmax = 240 nm, which is predominantly due to an intraligand charge transfer (ILCT), and another one at λmax = 332 nm is due to a ligand to ligand charge transfer (LLCT). The IR spectrum of compound 1 shown in Fig. 3S† confirms the presence of the organic ligands through the typical vibrations of benzene/pyridine aromatic rings, oxime and carboxylic groups.8c A broad band centered at 3375 cm−1 is due to the presence of water molecules. Bands characteristic for the 2-pyao ligand are at 1605 cm−1 (s, pyridyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1474 cm−1 (s, N–O). A strong doublet band (1562 and 1534 cm−1) is attributed to νasym(CO2), and a strong doublet band (1370 and 1346 cm−1) is attributed to νsym(CO2). Vibrational peaks in the region 810–680 cm−1 are assigned to C–H deformation modes.
N), 1474 cm−1 (s, N–O). A strong doublet band (1562 and 1534 cm−1) is attributed to νasym(CO2), and a strong doublet band (1370 and 1346 cm−1) is attributed to νsym(CO2). Vibrational peaks in the region 810–680 cm−1 are assigned to C–H deformation modes.
Crystallography
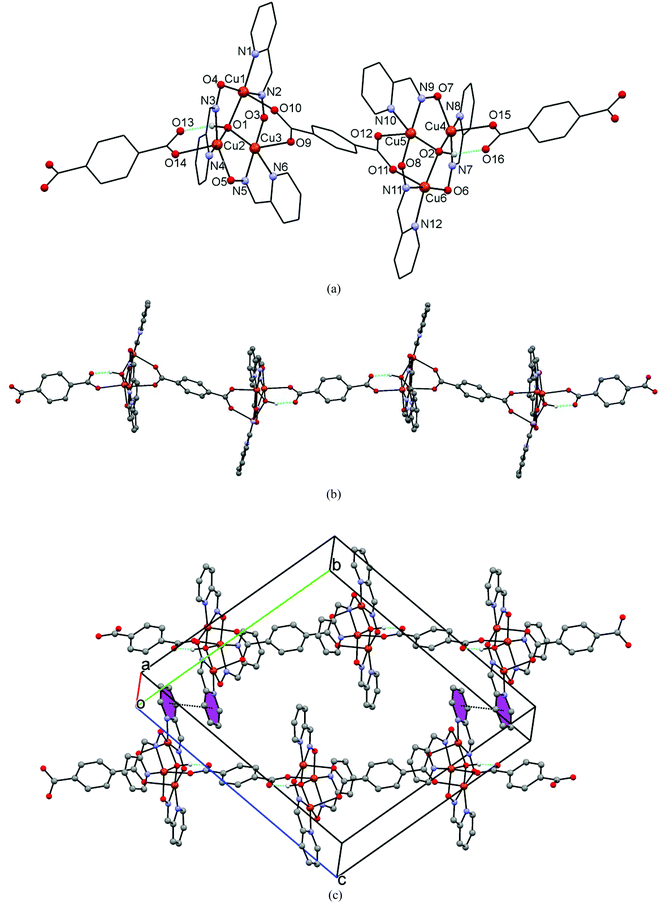
Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The asymmetric unit comprises two similar triangular [Cu3(μ3-OH)(2-pyao)3] cores bonded through two crystallographically different bdc2− ligands (Fig. 1a). The geometry around each of the copper(II) ions in the trimeric unit is best described as a distorted square pyramid having a N2O3 coordination environment. The degree of distortion for five-coordinated complexes is indicated by the general descriptor τ = (β − α)/60,20 where α and β are the two largest angles at the metal center. For the idealized square pyramidal extreme τ = 0. In compound 1τ values are in the range of 0.1–0.225. The trimeric skeletons are created by the pyridine N atom and the oximato nitrogen and oxygen atoms of three 2-pyao ligands, while the O atom of the μ3-OH ligand [O(1), O(2)] completes the square-planar bases of the three metal atoms, with Cu–O bond distances in the range of 1.940(4)–1.971(4) Å. The Cu3 triangles and the bdc2− ligands are assembled to form a one-dimensional polymer where two bdc2− molecules bridge the Cu(1,2,3) and Cu(4,5,6) units with alternating mono- and bidentate bridging modes (Fig. 1b). The slight asymmetry in each triangle is derived from this different bdc2− coordination on opposite sides of the trimeric units. Thereby, one coordinates in a bis-bidentate and another in a bis-monodentate mode while linking to different Cu(II) atoms. A similar asymmetry was also observed for other carboxylates with 2,4,5-trichlorophenoxy,8c acetato,13 benzoato,9,13 and perchlorato11a and nitrato21 ligands. The bis-bidentate bdc2− ligand bridges Cu(1) and Cu(3) [Cu(1)–O(10) = 2.252(4) Å, Cu(3)–O(9) = 2.245(4) Å], and Cu(5) and Cu(6) [Cu(5)–O(12) = 2.242(4) Å, Cu(6)–O(11) = 2.238(4) Å]. It alternates in the polymeric chain with the bis-monodentate ligand which bridges Cu(2) and Cu(4) [Cu(2)–O(14) = 2.306(4) Å and Cu(4)–O(15) = 2.267(4) Å]. All these oxygen atoms occupy the apical positions of the distorted square-base pyramids. In addition, hydrogen bonds are formed from the bis-monodentate ligand to the μ3-OH ligand of the metallacrown ring with O(1)−H(1)⋯O(13) = 1.67 Å [O⋯O = 2.604(5) Å] and O(2)–H(2)⋯O(16) = 1.67 Å [O⋯O = 2.591(5) Å]. The torsion angles between the Cu(II) ions across the oximato-bridges are −22.96° for Cu(1)–O(4)–N(3)–Cu(2), 5.59° for Cu(2)–O(5)–N(5)–Cu(3), −14.52° for Cu(3)–O(3)–N(2)–Cu(1), −3.04° for Cu(4)–O(7)–N(9)–Cu(5), 10.92° for Cu(5)–O(8)–N(11)–Cu(6), and 24.92° for Cu(6)–O(6)–N(7)–Cu(4). The central μ3-OH ligands are placed 0.603 and 0.613 Å above the Cu(1,2,3) and Cu(4,5,6) planes, respectively. The Cu3 units form scalene triangles, however they are close to equilateral triangles with Cu(1)⋯Cu(2), Cu(2)⋯Cu(3), Cu(1)⋯Cu(3) distances of 3.229, 3.279, and 3.154 Å, and Cu(4)⋯Cu(5), Cu(5)⋯Cu(6), and Cu(4)⋯Cu(6) distances of 3.267, 3.154 and 3.255 Å, respectively. The Cu3 triangles are situated in approximately parallel planes as the interplanar angle of 3.11° between the Cu3 cores indicates. The shortest Cu⋯Cu separations across the bis-bidentate bridging bdc2− ligand are 11.296 and 11.350 Å, while across the bis-monodentate bridging ligand it is 11.830 Å.
. The asymmetric unit comprises two similar triangular [Cu3(μ3-OH)(2-pyao)3] cores bonded through two crystallographically different bdc2− ligands (Fig. 1a). The geometry around each of the copper(II) ions in the trimeric unit is best described as a distorted square pyramid having a N2O3 coordination environment. The degree of distortion for five-coordinated complexes is indicated by the general descriptor τ = (β − α)/60,20 where α and β are the two largest angles at the metal center. For the idealized square pyramidal extreme τ = 0. In compound 1τ values are in the range of 0.1–0.225. The trimeric skeletons are created by the pyridine N atom and the oximato nitrogen and oxygen atoms of three 2-pyao ligands, while the O atom of the μ3-OH ligand [O(1), O(2)] completes the square-planar bases of the three metal atoms, with Cu–O bond distances in the range of 1.940(4)–1.971(4) Å. The Cu3 triangles and the bdc2− ligands are assembled to form a one-dimensional polymer where two bdc2− molecules bridge the Cu(1,2,3) and Cu(4,5,6) units with alternating mono- and bidentate bridging modes (Fig. 1b). The slight asymmetry in each triangle is derived from this different bdc2− coordination on opposite sides of the trimeric units. Thereby, one coordinates in a bis-bidentate and another in a bis-monodentate mode while linking to different Cu(II) atoms. A similar asymmetry was also observed for other carboxylates with 2,4,5-trichlorophenoxy,8c acetato,13 benzoato,9,13 and perchlorato11a and nitrato21 ligands. The bis-bidentate bdc2− ligand bridges Cu(1) and Cu(3) [Cu(1)–O(10) = 2.252(4) Å, Cu(3)–O(9) = 2.245(4) Å], and Cu(5) and Cu(6) [Cu(5)–O(12) = 2.242(4) Å, Cu(6)–O(11) = 2.238(4) Å]. It alternates in the polymeric chain with the bis-monodentate ligand which bridges Cu(2) and Cu(4) [Cu(2)–O(14) = 2.306(4) Å and Cu(4)–O(15) = 2.267(4) Å]. All these oxygen atoms occupy the apical positions of the distorted square-base pyramids. In addition, hydrogen bonds are formed from the bis-monodentate ligand to the μ3-OH ligand of the metallacrown ring with O(1)−H(1)⋯O(13) = 1.67 Å [O⋯O = 2.604(5) Å] and O(2)–H(2)⋯O(16) = 1.67 Å [O⋯O = 2.591(5) Å]. The torsion angles between the Cu(II) ions across the oximato-bridges are −22.96° for Cu(1)–O(4)–N(3)–Cu(2), 5.59° for Cu(2)–O(5)–N(5)–Cu(3), −14.52° for Cu(3)–O(3)–N(2)–Cu(1), −3.04° for Cu(4)–O(7)–N(9)–Cu(5), 10.92° for Cu(5)–O(8)–N(11)–Cu(6), and 24.92° for Cu(6)–O(6)–N(7)–Cu(4). The central μ3-OH ligands are placed 0.603 and 0.613 Å above the Cu(1,2,3) and Cu(4,5,6) planes, respectively. The Cu3 units form scalene triangles, however they are close to equilateral triangles with Cu(1)⋯Cu(2), Cu(2)⋯Cu(3), Cu(1)⋯Cu(3) distances of 3.229, 3.279, and 3.154 Å, and Cu(4)⋯Cu(5), Cu(5)⋯Cu(6), and Cu(4)⋯Cu(6) distances of 3.267, 3.154 and 3.255 Å, respectively. The Cu3 triangles are situated in approximately parallel planes as the interplanar angle of 3.11° between the Cu3 cores indicates. The shortest Cu⋯Cu separations across the bis-bidentate bridging bdc2− ligand are 11.296 and 11.350 Å, while across the bis-monodentate bridging ligand it is 11.830 Å.
The structure of the 1D polymer 1 was refined as a hydrate with the overall uptake of six disordered water molecules per formula unit. The molecular packing was analyzed by the Mercury program.18 It indicates negligible solvent accessible voids (SAVs) for the hydrated structure (2.3% of the unit cell volume calculated with the accommodated water molecules). The SAVs for the solvent-free network give a value of 531.4 Å3 (∼16.8% of the unit cell volume), thus indicating the high solvent uptake. The packing of the coordination polymers reveals π-stacking interactions between a pair of inversion-related 2-pyao pyridine rings (Fig. 1c). One might speculate that a similar stacking between the next pairs of 2-pyao-rings is precluded by the water accommodation in the crystal lattice situated in the hydrophilic regions in proximity to the carboxylic groups (Fig. 4S†).
Thermal stability and sorption properties
To estimate the thermal stability of 1, a thermogravimetric analysis (TGA) was undertaken. The TGA plots indicate at least a four-stage decomposition process in an air atmosphere (Fig. 5S†). In the temperature range of 25–160 °C, two maxima are observed and assigned to the solvent water loss. This gradual loss corresponds to the removal of four water molecules in the temperature range of 25–105 °C (8.40%), and two water molecules in the range of 105–160 °C (4.05%). This step-wise process is explained by the different modes of water association in the crystal, in line with the reported data for the hydrated mixed-ligand Cu(II) terephthalates.22 The sample mass decreases by 12.45% (calculated 12.80%). The dehydrated sample remains stable in the temperature range of 160–236 °C. At 236 °C a rapid decomposition of the compound starts, resulting in a loss of 48.8% by weight. The decomposition is accompanied by a strong exothermic effect. Although decarboxylation and CO removal is the most favorable process for this stage,22 the significant weight loss indicates that fragmentation is not so simple and that decomposition steps are strongly overlapped and include also the degradation of the Cu3 core.23 The final thermal degradation with a loss of 11.06% mass is also accompanied by an exothermic heat effect. From 354 °C on, the degradation product (possibly copper(II) oxide) is stable up to 1100 °C. The total loss is 73.20% (calculated 71.56%).The N2 sorption isotherm was recorded for the dehydrated 1 (Fig. 6S†). The compound hardly adsorbs N2 at 77 K, which leads to the insignificant BET (Brunauer–Emmett–Teller) surface area of 9.9 m2 g−1. Near saturation pressure, there is a sudden increase in sorption caused by condensation of nitrogen; this can be explained by the presence of mesopores. The total pore volume constitutes 0.046 cm3 g−1. As it is evidenced from Fig. 6S,† the hysteresis loop is caused by these mesopores.
Magnetic properties and structure–property correlation
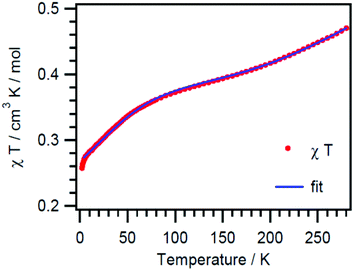
Compound 1 was studied by magnetic susceptibility measurements. The χmT(T) plot (Fig. 2) of a polycrystalline sample of 1 shows a room temperature value of 0.47 cm3 K mol−1 per Cu3 which is much lower than that expected for three uncoupled spins with S = 1/2; this is an evidence for strong antiferromagnetic coupling. Upon cooling, the χmT values reach a declining plateau of about 0.4 cm3 K mol−1 until they decrease again at temperatures below 80 K to reach 0.26 cm3 K mol−1 at 1.9 K. At the plateau, the χmT values of about 0.4 cm3 K mol−1 correspond to those of an isolated S = 1/2 ground state with an usual g value for Cu(II). A strikingly similar behavior was observed for triangular μ3-OH/oximato Cu(II)3 complexes in a recent study by Escuer et al.14a The magnetization data taken at 1.9 K confirm the S = 1/2 ground state of the Cu3 unit (see Fig. 7S†).It is well known that for an equilateral triangular Cu(II)3 complex, the isotropic exchange interaction yields two degenerate spin doublets (2E) as the ground state, separated from a spin quadruplet (4A) state through an energy gap of 3J/2. This result is directly deduced from the isotropic exchange Hamiltonian: Hiso = −J12S1·S2 − J13S1·S3 − J23S2·S3, with J12 = J13 = J23 = J for the equilateral case.
Approximating a trinuclear system with an isosceles triangle (thus, the closer Cu(II) ions are treated as equivalent) gives J = J12 = J23 ≠ J13 = j and δ = J − j. At this level of theory, the high temperature behavior including the temperature region representing the plateau of the χmT values can be analyzed. However, following the discussions in the work of Escuer et al.14a and Ferrer et al.,14b the magnetic behavior at low temperatures is attributed to antisymmetric exchange (ASE) interactions within the trimeric units. The corresponding Hamiltonian is described by
| HASE = G12[S1 × S2] + G23[S2 × S3] + G31[S3 × S1] |
A magnetostructural correlation can be discussed based on the actual structural data and the literature data. Table 3 summarizes geometric parameters and J values for the complexes containing a Cu3-oximato coordination core. It is evident, that geometric parameters such as Cu⋯Cu and Cu⋯μ3-OH distances alter in narrow intervals, while the μ3-OH distance from the Cu3 plane varies in the range of 0.393–0.695 Å. The reported 0D complexes reveal higher J values than polymeric ones. Among the polymeric materials the J value is not significantly influenced by the inter-core interaction since it is rather weak, as Chakraborty et al. have estimated it.8b
| Compound | Topology | O(H) distance from the Cu3 plane/Å | Cu–O(H)/Å (av) | Cu–Cu′/Å (av) | −J/cm−1 | Reference |
|---|---|---|---|---|---|---|
| a BAQXUO = catena-((μ3-hydroxo)-tris(μ2-N-oxy-1-phenyl-1-(pyridin-2-yl) methanimine)-(μ2-dicyanamide)-(acetato)-tri-copper(II)); IMIXAE = catena-(bis(μ3-hydroxo)-bis(μ3-N-oxy-1-(pyrazin-2-yl)ethanimine)-tetrakis(μ2-N-oxy-1-(pyrazin-2-yl)ethanimine)-bis(methanol)-bis(nitrato-O)-hexa-copper dinitrate); IMIXIM = catena-(bis(μ3-N-oxy-1-(pyrazin-2-yl)ethanimine)-(μ3-oxo)-di-copper bis(tetrafluoro-borate)); OXMCUB = bis((μ4-oxo)-tris(μ2-1,2-diphenyl-2-(methylimino)-1-ethanone-oximato-N,N′,O)-tri-copper(II)) diperchlorate; HOHQUR = (μ3-hydroxo)-tris(μ2-N-oxy-1,1-diphenylmethanimine-N,N′,O)-(μ2-(2,4,5-trichlorophenoxy)acetato-O,O′)-((2,4,5-trichloro-phenoxy)acetato-O)-tri-copper(II) methanol solvate; ILEGEM = (μ3-hydroxo)-tris(μ2-phenyl(pyridin-2-yl)methanone oximato-N,N′,O)-(methanol-O)-(nitrato-O)-tri-copper(II) nitrate; RIQPOY = (μ3-hydroxo)-(μ2-benzoato-O,O′)-tris(μ2-6-methyl-2-pyridylaldoximato)-(benzoato-O)-tri-copper; RIQQIT = bis(μ3-hydroxo)-hexakis(μ2-([(6-methylpyridin-2-yl)methylidene]amino)oxidanide)-tris(μ2-perchlorato)-hexa-copper perchlorate; RIQQOZ = bis(μ3-hydroxo)-hexakis(μ2-([(6-methylpyridin-2-yl)methylidene]amino)oxidanide)-tris(μ2-nitrato)-hexa-copper(II) nitrate monohydrate. | ||||||
| 1 | 1D | 0.612/0.622 | 1.961 | 3.220/3.226 | 430 | Present work |
| BAQXUO | 1D | 0.616 | 1.957 | 3.215 | 460 | 8b |
| IMIXAE | 1D | 0.649/0.599 | 1.967/1.954 | 3.215/3.221 | 447 | 13 |
| IMIXIM | 3D | 0.393 | 1.887 | 3.196 | 298 | 13 |
| OXMCUB | 0D | 0.695 | 1.964 | 3.194 | 122 | 11b |
| HOHQUR | 0D | 0.66 | 1.942 | 3.166 | 220 | 8c |
| ILEGEM | 0D | 0.549 | 1.934 | 3.212 | 411 | 8f |
| RIQPOY | 0D | 0.521 | 1.972 | 3.289 | 615 | 14 |
| RIQQIT | 0D | 0.545/0.576 | 1.982/1.992 | 3.300/3.303 | 621 | 14 |
| RIQQOZ | 0D | 0.588/0.587 | 1.972/1.979 | 3.258/3.268 | 683 | 14 |
Conclusions
In conclusion, the first example of a coordination polymer comprising Cu3-metallacrowns and 1,4-benzenedicarboxylate bridges is reported. The simple and direct synthetic route employing oxime and dicarboxylate ligands provides a direct access to the polymeric materials composed of paramagnetic Cu3 units representing spin-frustrated metallacrowns.Acknowledgements
The authors acknowledge the financial support from the project SCOPES (IZ73Z0_152404/1).Notes and references
- (a) G. Mezei, C. M. Zaleski and V. L. Pecoraro, Chem. Rev., 2007, 107, 4933–5003 CrossRef CAS PubMed; (b) E. Zangrando, M. Casanova and E. Alessio, Chem. Rev., 2008, 108, 4979–5013 CrossRef CAS PubMed.
- (a) E. I. Solomon, U. M. Sundaram and T. E. Machonkin, Chem. Rev., 1996, 96, 2563–2606 CrossRef CAS PubMed; (b) R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239–2314 CrossRef CAS; (c) W. Kaim and J. Rall, Angew. Chem., Int. Ed. Engl., 1996, 35, 43–60 CrossRef CAS.
- (a) N. Kitajima and Y. Moro-oka, Chem. Rev., 1994, 94, 737–757 CrossRef CAS; (b) E. I. Solomon, F. Tuczek, D. E. Root and C. A. Brow, Chem. Rev., 1994, 94, 827–856 CrossRef CAS; (c) Y.-B. Jiang, H.-Z. Kou, R.-J. Wang, A.-L. Cui and J. Ribas, Inorg. Chem., 2005, 44, 709–715 CrossRef CAS PubMed; (d) T. C. Stamatatos, J. C. Vlahopoulou, Y. Sanakis, C. P. Raptopoulou, V. Psycharis, A. K. Boudalis and S. P. Perlepes, Inorg. Chem. Commun., 2006, 9, 814–818 CrossRef CAS PubMed; (e) J. Yoon, L. M. Mirica, T. D. P. Stack and E. I. Solomon, J. Am. Chem. Soc., 2005, 127, 13680–13693 CrossRef CAS PubMed; (f) A. Messerschmidt, Struct. Bonding, 1998, 90, 37–68 CrossRef CAS.
- (a) L. Gutierrez, G. Alzuet, J. A. Real, J. Cano, J. Borras and A. Castineiras, Inorg. Chem., 2000, 39, 3608–3614 CrossRef CAS; (b) O. Kahn, Chem. Phys. Lett., 1997, 265, 109–114 CrossRef CAS; (c) S. Ferrer, J. G. Haasnoot, J. Reedijk, E. Muller, M. B. Cingi, M. Lanfranchi, A. M. M. Lanfredi and J. Ribas, Inorg. Chem., 2000, 39, 1859–1867 CrossRef CAS; (d) X. Liu, M. P. de Miranda, E. J. L. McInnes, C. A. Kilner and M. A. Halcrow, Dalton Trans., 2004, 59–64 RSC; (e) L. M. Mirica and T. D. P. Stack, Inorg. Chem., 2005, 44, 2131–2133 CrossRef CAS PubMed; (f) J. Yoon and E. I. Solomon, Coord. Chem. Rev., 2007, 251, 379–400 CrossRef CAS PubMed; (g) G. Pascu, C. Deville, S. E. Clifford, L. Guenée, C. Besnard, K. W. Krämer, S.-X. Liu, S. Decurtins, F. Tuna, E. J. L. McInnes, R. E. P. Winpenny and A. F. Williams, Dalton Trans., 2014, 43, 656–662 RSC; (h) A. S. Degtyarenko, M. Handke, K. W. Krämer, S.-X. Liu, S. Decurtins, E. B. Rusanov, L. K. Thompson, H. Krautscheid and K. V. Domasevitch, Dalton Trans., 2014, 43, 8530–8542 RSC; (i) O. Kahn, Molecular Magnetism, VCH, Weinheim, Germany, 1993 Search PubMed; (j) M. Pilkington and S. Decurtins, Chimia, 2000, 54, 593–601 CAS; (k) In Magnetism – Molecular and Supramolecular Perspectives, Coord. Chem. Rev., ed.L. K. Thompson, 2005, 249, 2549–2730.
- (a) P. Chaudhuri, Coord. Chem. Rev., 2003, 243, 143–190 CrossRef CAS; (b) C. J. Milios, T. C. Stamatatos and S. P. Perlepes, Polyhedron, 2006, 25, 134–194 CrossRef CAS PubMed.
- (a) R. Beckett and B. F. Hoskins, J. Chem. Soc., Dalton Trans., 1972, 291–295 RSC.
- A. Escuer, G. Vlahopoulou, S. P. Perlepes and F. A. Mautner, Inorg. Chem., 2011, 50, 2468–2478 CrossRef CAS PubMed.
- (a) T. Afrati, C. M. Zaleski, C. Dendrinou-Samara, G. Mezei, J. W. Kampf, V. L. Pecoraro and D. P. Kessissoglou, Dalton Trans., 2007, 2658–2668 RSC; (b) A. Chakraborty, K. L. Gurunatha, A. Muthulakshmi, S. Dutta, S. K. Pati and T. K. Maji, Dalton Trans., 2012, 41, 5879–5888 RSC; (c) T. Afrati, C. Dendrinou-Samara, C. Raptopoulou, A. Terzis, V. Tangoulis, A. Tsipis and D. P. Kessissoglou, Inorg. Chem., 2008, 47, 7545–7555 CrossRef CAS PubMed; (d) G.-X. Liu, H.-M. Xu and X.-M. Ren, Chin. J. Struct. Chem., 2010, 29, 1072–1076 CAS; (e) T. Afrati, A. A. Pantazaki, C. Dendrinou-Samara, C. Raptopoulou, A. Terzis and D. P. Kessissoglou, Dalton Trans., 2010, 39, 765–775 RSC; (f) G.-X. Liu, W. Guo, S. Nishihara and X.-M. Ren, Inorg. Chim. Acta, 2011, 368, 165–169 CrossRef CAS PubMed.
- T. C. Stamatatos, J. C. Vlahopoulou, Y. Sanakis, C. P. Raptopoulou, V. Psycharis, A. K. Boudalis and S. P. Perlepes, Inorg. Chem. Commun., 2006, 9, 814–818 CrossRef CAS PubMed.
- (a) E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2006, 45, 7722–7725 CrossRef CAS PubMed; (b) W. Quellette, M. H. Yu, C. J. O'Connor, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 2006, 45, 3497–3500 CrossRef PubMed; (c) B. Ding, L. Yi, P. Cheng, D. Z. Liao and S. P. Yan, Inorg. Chem., 2006, 45, 5799–5803 CrossRef CAS PubMed; (d) M. Casarin, C. Corvaja, C. Di Nicola, D. Falcomer, L. Franco, M. Monari, L. Pandolfo, C. Pettinari, F. Piccinelli and P. Tagliatesta, Inorg. Chem., 2004, 43, 5865–5876 CrossRef CAS PubMed; (e) Y. Z. Zheng, M. L. Tong, W. Xue, W. Zhang, X. M. Chen, F. Grandjean and G. J. Long, Angew. Chem., Int. Ed., 2007, 46, 6076–6080 CrossRef CAS PubMed; (f) P. Albores and E. Rentschler, Inorg. Chem., 2008, 47, 7960–7962 CrossRef CAS PubMed.
- (a) Y. Agnus, R. Louis, B. Metz, C. Boudon, J. P. Gisselbrecht and M. Gross, Inorg. Chem., 1991, 30, 3155–3161 CrossRef CAS; (b) R. J. Butcher, C. J. O'Connor and E. Sinn, Inorg. Chem., 1981, 20, 537–545 CrossRef CAS.
- (a) D. Maity, P. Mukherjee, A. Ghosh, M. G. B. Drew, C. Diaz and G. Mukhopadhyay, Eur. J. Inorg. Chem., 2010, 807–813 CrossRef CAS; (b) S. Karmakar, O. Das, S. Ghosh, E. Zangrando, M. Johann, E. Rentschler, T. Weyhermuller, S. Khanra and T. K. Paine, Dalton Trans., 2010, 39, 10920–10927 RSC; (c) L. K. Das, M. G. B. Drew, C. Diaz and A. Ghosh, Dalton Trans., 2014, 43, 7589–7598 RSC.
- A. Escuer, B. Cordero, M. Font-Bardia and T. Calvet, Inorg. Chem., 2010, 49, 9752–9754 CrossRef CAS PubMed.
- (a) A. Escuer, G. Vlahopoulou, F. Lloret and F. A. Mautner, Eur. J. Inorg. Chem., 2014, 83–92 CrossRef CAS; (b) S. Ferrer, F. Lloret, E. Pardo, J. M. Clemente-Juan, M. Liu-Gonzalez and S. Garcia-Granda, Inorg. Chem., 2012, 51, 985–1001 CrossRef CAS PubMed.
- (a) Y.-Z. Zheng, Z. Zheng and X.-M. Chen, Coord. Chem. Rev., 2014, 258–259, 1–15 CrossRef CAS PubMed; (b) B. Dey, A. Das, S. R. Choudhury, A. D. Jana, L.-P. Lu, M.-L. Zhu and S. Mukhopadhyay, Inorg. Chim. Acta, 2010, 363, 981–987 CrossRef CAS PubMed; (c) X. Xu, Y. Ma and E. Wang, J. Solid State Chem., 2007, 180, 3136–3145 CrossRef CAS PubMed; (d) R. A. Sarmiento-Perez, L. M. Rodriguez-Albelo, A. Gomez, M. Autie-Perez, D. W. Lewis and A. R. Ruiz-Salvador, Microporous Mesoporous Mater., 2012, 163, 186–191 CrossRef CAS PubMed.
- (a) L. Croitor, E. B. Coropceanu, A. V. Siminel, A. E. Masunov and M. S. Fonari, Polyhedron, 2013, 60, 140–150 CrossRef CAS PubMed; (b) L. Croitor, E. B. Coropceanu, A. E. Masunov, H. J. Rivera-Jacquez, A. V. Siminel, V. I. Zelentsov, T. Ya. Datsko and M. S. Fonari, Cryst. Growth Des., 2014, 14, 3935–3948 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- (a) A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC; (b) E. Melnic, E. B. Coropceanu, O. V. Kulikova, A. V. Siminel, D. Anderson, H. J. Rivera-Jacquez, A. E. Masunov, M. S. Fonari and V. Ch. Kravtsov, J. Phys. Chem. C, 2014, 118, 30087–30100 CrossRef CAS.
- B. Cordero, A. Escuer, M. Font-Bardia and T. Calvet, Polyhedron, 2013, 64, 84–90 CrossRef CAS PubMed.
- J. Rogan and D. Poleti, Thermochim. Acta, 2004, 413, 227–234 CrossRef CAS PubMed.
- R. Bucci, V. Carunchio, A. D. Magri and A. L. Magri, J. Therm. Anal., 1984, 29, 679–686 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-Vis, IR, TGA, DSC, ad/desorption plots, magnetization data, figures of as-grown crystals and crystal packing. CCDC 1047595. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt00533g |
| This journal is © The Royal Society of Chemistry 2015 |