Synthesis, structures and magnetic properties in 3d-electron-rich isostructural complexes based on chains with sole syn–anti carboxylate bridges†
Abstract
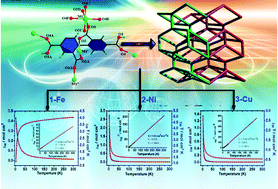
To evaluate magnetic properties of isostructural compounds, a series of 3D carboxylate coordination polymers [M(H2bpta)]n, (H4bpta = 2,2′,4,4′-biphenyltetracarboxylic acid, M = Fe(II) (1), Ni(II) (2), Cu(II) (3) and Zn(II) (4)), was synthesized in H2O–CH3CN or H2O solvents, respectively. Structurally, complexes 1–4 have isostructural features with (5,5)-connected 3D framework, wherein the M(II) centre takes an octahedral coordination environment consisting of six oxygen atoms from carboxylates of ligands. The M(II) sites are linked by syn–anti carboxylates to form chains with an M⋯M separation of 4.880(2) (M = Fe), 4.784(2) (M = Ni), 4.541(2) (M = Cu), and 4.607(2) Å (M = Zn), respectively. The shortest M⋯M distances between interchains locate 9.122(4), 9.077(3), 9.361(3), and 8.767(2) Å, respectively. Magnetically, the isostructural polymers show different magnetic behaviors due to different spins of central ions. Theoretical analysis indicates that couplings between magnetic ions obey uniform chain models. The magnetic susceptibility of 1 and 2 are perfectly fitted by the modified Fisher model to yield an effective intra-chain exchange coupling constant of −0.81(1) and 3.67(2) cm−1, respectively. For 3, a Heisenberg ferromagnetic S = 1/2 chain included the intra-chain magnetic exchange interaction (J = 9.28(1) cm−1, and zj′ = −0.068(3) cm−1), weak ferromagnetic interactions in intra-chains, and weak antiferromagnetic interactions between interchains. The phenomena of 1–3 accord with the common view that the exchange interaction between two magnetic M(II) ions bridged by the syn–anti carboxylate bridge is dominantly weak ferro- or anti-ferromagnetic interactions. In addition, the M–O–C–O–M spin exchange interactions |J| of M2(CO2)2 (M = Mn(3d5)20, Fe(3d6), Co(3d7)20, Ni(3d8), Cu(3d9)) decrease in strength with Cu2(CO2)2 > Ni2(CO2)2 > Co2(CO2)2 > Fe2(CO2)2 > Mn2(CO2)2, consistent with orbit order.

 Please wait while we load your content...
Please wait while we load your content...