Open Access Article
Open Access ArticleChirality detection of two enantiomorphic 3D lanthanide coordination polymers by vibrational circular dichroism spectra†
Xiu-Ying
Zheng
,
Han
Zhang
,
Ling-Yun
Cao
,
Xiang-Jian
Kong
*,
La-Sheng
Long
* and
Lan-Sun
Zheng
State Key Laboratory of Physical Chemistry of Solid Surface and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China. E-mail: xjkong@xmu.edu.cn; lslong@xmu.edu.cn
First published on 12th February 2015
Abstract
Two enantiomorphic 3D lanthanide coordination polymers of {[Dy5(L)4(H2O)10][Dy(H2O)7][Na(H2O)5]}·(ClO4)7·(H2O)15 (1a for R and 1b for S) with chiral helical chains were synthesized based on an achiral ligand N-(2-hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid (H3L) and Dy(ClO4)3. Crystal analysis revealed that 1a and 1b were crystallized in chiral space groups P4132 and P4332, respectively. The absolute configurations of the two structures were evidenced by vibrational circular dichroism (VCD) spectra with one single crystal sample.
Chiral lanthanide coordination polymers have attracted much attention because of their potential applications in chiral sensors, chiral resolution, and chiral catalysis.1–3 Up to now, a number of chiral lanthanide coordination polymers have been obtained by adopting chiral agents.2 However, the construction of chiral lanthanide coordination polymers from achiral starting materials remains a great challenge.3
It is well known that determination of the absolute configuration is crucial in understanding the functionality of a chiral molecule.4–6 At present, electronic circular dichroism (ECD) is the primary spectroscopic method for the detection of absolute configuration of chiral molecules. However, ECD spectra require chiral molecules that have electronic absorption in the UV-visible regions. If the chiral molecule has no chromophore group, the detection of absolute configuration will be difficult by the conventional ECD methods.
In recent years, vibrational circular dichroism (VCD) has become a new powerful tool for determination of the absolute configurations of chiral molecules.7–12 Compared with the ECD spectra, the VCD has many advantages. First, VCD is the extension of ECD into the infrared and near-infrared regions. The absorption spectrum of ECD is caused by the molecular electronic energy level transition, while VCD reveals the circularly polarized molecular vibration transition of IR radiation. Even though a molecule has no signals in ECD spectra, it can be a target for VCD.9 Second, VCD provides more detailed information based on the stereochemical characters of chiral molecules, compared with ECD.8c Third, the theoretical of VCD spectra can be calculated based on practical analyses of chiral molecules.10
VCD has been investigated for the studies of conformation information of chiral molecules in solution, in particular with recent developments in the study of solute–solvent interactions such as the chirality transfer and biomolecular probe.8c,10 Although in the last few years, the solid VCD measurement has been applied in the characterization of chiral transition metal polymers,11 the investigation of absolute configurations of chiral lanthanide coordination polymers by solid VCD spectra is rare.12 Recently, a lanthanide induced VCD enhancement effect was observed,12a which may give rise to the developments of chiral lanthanide coordination polymers.
In this work, we have reported two enantiomorphic 3D chiral lanthanide coordination polymers, formulated as {[Dy5(L)4(H2O)10][Dy(H2O)7][Na(H2O)5]}·(ClO4)7·(H2O)15 (1a for R and 1b for S), based on an achiral ligand N-(2-hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid (H3L) and Dy(ClO4)3, in 10 mL deionized water [ESI†]. Single crystal X-ray analysis revealed that 1a was crystallized in the chiral space group P4132, while 1b was crystallized in P4332. The absolute configuration of 1a and 1b was also investigated by VCD spectra with one single crystal sample.
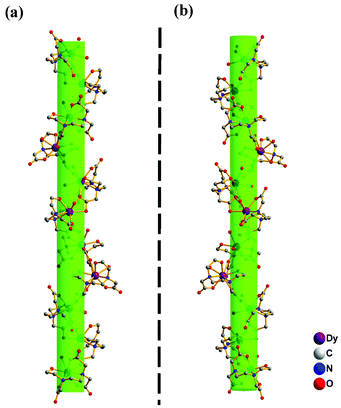
As a representative, compound 1a is described below to illustrate the structural features. Single crystal structure analysis reveals that the asymmetrical unit of 1a consists of two independent Dy3+, one disordered [Dy(H2O)7]0.5[Na(H2O)5]0.5 unit, two L3− ligand anions, three and half ClO4−, five aqua ligands, and 7.5 guest water molecules. For Dy1 and Dy2, each of them is coordinated by a chelating L3− ligand in a six-coordinated manner with four O atoms and two N atoms from the L3− ligand, forming a [DyL] unit, as shown in Fig. 1a. Completing the coordination geometry of Dy1 and Dy2 respectively with one and two terminal aqua ligands, results in [DyL(H2O)] and [DyL(H2O)2] units. The [DyL(H2O)] and [DyL(H2O)2] units are connected alternately, generating a chiral helical chain structure of [Dy2(L)2(H2O)3]n (Fig. 2a). The 3D structure of 1a can be viewed as three kinds of adjacent chiral chains in three dimensional directions that are linked to each other by coordinating Dy3 with four carboxylate groups from four [DyL(H2O)2] units, as shown in Fig. 1b and 2b. The present compound is the first 3D lanthanide coordination polymer based on the N-(2-hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid. Each disordered Dy4 and Na1, in the half-occupancy [Dy(H2O)7]3+ and [Na(H2O)5] form, is connected to each Dy1. Dy1 and Dy2 are nona-coordinated and form the tri-capped trigonal prism and capped square-antiprism coordination geometry, while Dy3 and Dy4 are octa-coordinated and located in the centre of the di-capped trigonal prism.
 | ||
| Fig. 1 (a) Ball and stick view of the coordination model for Dy1 and Dy2; (b) ball and stick view of the coordination model for Dy3. | ||
Compound 1b is the enantiomorphism of compound 1a. Differing from 1a, in which, the chiral chains are generated around the 41 axis, and the chiral chains in 1b are generated around the 43 axis, as shown in Fig. 3. The Dy–O and Dy–N distances of 1 range from 2.270(6) to 2.717(7) Å and 2.609(9)–2.680(10) Å (Tables S2 and S3†), which are comparable to those in reported Dy-compounds.12b
To further investigate the absolute configurations of the two enantiomers, the solid-state ECD spectra (KCl pellet) based on one single crystal were measured. However, no significant ECD signals are observed in 200–800 nm. Thus, the powerful solid-state VCD spectra were studied in the wavenumber region 1400–1800 cm−1. Fig. 4 shows the observed VCD and IR spectra for 1a and 1b based on one single crystal sample. The VCD signals at about 1650 and 1630 cm−1 are assigned to the asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching vibrations of the carboxy group in the ligand.13 The VCD signals at 1590, 1580, 1560 and 1540 cm−1 are attributable to the stretching vibrations of the C–C in the L3−.14 The distinct VCD signals indicated that the crystals of 1 exists in two kinds of enantiomorphism isomers, R for P4132 and S for P4332.
O and C–O stretching vibrations of the carboxy group in the ligand.13 The VCD signals at 1590, 1580, 1560 and 1540 cm−1 are attributable to the stretching vibrations of the C–C in the L3−.14 The distinct VCD signals indicated that the crystals of 1 exists in two kinds of enantiomorphism isomers, R for P4132 and S for P4332.
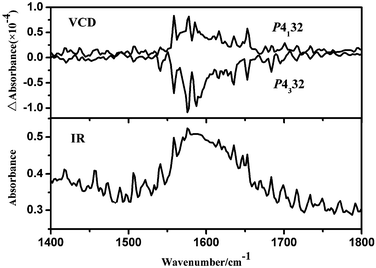 | ||
| Fig. 4 VCD (top) and IR absorption (bottom) spectra of 1a and 1b based on a pressed KBr disk of one single crystal sample. | ||
The temperature dependence of magnetic susceptibilities of 1 was measured from 2 to 300 K with 1000 Oe. The χMT and χM−1vs. T curves are displayed in Fig. S7.† At 300 K, the χMT value was found to be 82.57 cm3 K mol−1, which is consistent with the calculated spin-only values of 85.02 cm3 K mol−1 for 6 uncorrelated Dy3+ cations (6H15/2, g = 4/3). The χMT value remains roughly constant from 300 to 140 K. On further cooling to 2 K, the χMT value slowly decreases to 59.62 cm3 K mol−1. The curve of χM−1vs. T obeys the Curie–Weiss law with C = 84.96 cm3 K mol−1, θ = −4.12 K. The profile of the χMT vs. T and the negative value suggest the presence of some antiferromagnetic interactions and possibly magnetic anisotropy/ligand fields.15 The field dependence of magnetization is shown in Fig. S8.† At 2 K, the magnetization firstly increases rapidly below 1.0 T and then slowly increases almost linearly without saturation at 7 T, suggesting the presence of significant anisotropy and/or low lying excited states.15
In summary, a pair of chiral enantiomorphic lanthanide coordination polymers 1a and 1b has been obtained based on achiral ligands. Structural analysis reveals that 1a and 1b were crystallized in chiral space groups P4132 and P4332, respectively. The absolute configurations of the enantiomorphism isomers were also detected by the VCD spectra. This work proved that the VCD spectra approach is a versatile method to explore the conformation of chiral lanthanide coordination polymers.
Acknowledgements
This work was supported by the 973 project (grant 2012CB821704 and 2014CB845601) from the Ministry of Science and Technology of China, the National Natural Science Foundation of China (grants no. 21422106, 21371144, 21431005 and 21390391), and the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201219) for financial support.Notes and references
- (a) R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673 RSC; (b) S. J. Bradberry, A. J. Savyasachi, M. Martinez-Calvo and T. Gunnlaugsson, Coord. Chem. Rev., 2014, 273–274, 226 CrossRef CAS PubMed; (c) H. Tsukube and S. Shinoda, Chem. Rev., 2002, 102, 2389 CrossRef CAS PubMed; (d) H. C. Aspinall, Chem. Rev., 2002, 102, 1807 CrossRef CAS PubMed.
- (a) J. Yuasa, T. Ohno, K. Miyata, H. Tsumatori, Y. Hasegawa and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9892 CrossRef CAS PubMed; (b) X. J. Kong, Y. L. Wu, L. S. Long, L. S. Zheng and Z. P. Zheng, J. Am. Chem. Soc., 2009, 131, 6918 CrossRef CAS PubMed.
- (a) X. H. Yan, Z. H. Cai, C. L. Yi, W. S. Liu, M. Y. Tan and Y. Tang, Inorg. Chem., 2011, 50, 2346 CrossRef CAS PubMed; (b) M. L. Sun, J. A. Zhang, Q. P. Lin, P. X. Lin and Y. G. Yao, Inorg. Chem., 2010, 49, 9257 CrossRef CAS PubMed.
- (a) G. L. Hamilton, E. J. Kang, M. Mba and F. D. Toste, Science, 2007, 317, 496 CrossRef CAS PubMed; (b) L. Q. Ma, J. M. Falkowski, C. Abney and W. B. Lin, Nat. Chem., 2010, 2, 838 CrossRef CAS PubMed.
- (a) M. Albrecht, Chem. Rev., 2001, 101, 3457 CrossRef CAS PubMed; (b) D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2009, 38, 273 CrossRef PubMed.
- (a) C. Schotes, M. Althaus, R. Aardoom and A. Mezzetti, J. Am. Chem. Soc., 2012, 134, 1331 CrossRef CAS PubMed; (b) S.-T. Wu, Z.-W. Cai, Q.-Y. Ye, C.-H. Weng, X.-H. Huang, X.-L. Hu, C.-C. Huang and N.-F. Zhuang, Angew. Chem., Int. Ed., 2014, 53, 12860 CrossRef CAS PubMed.
- (a) J. Sadlej, J. C. Dobrowolski and J. E. Rode, Chem. Soc. Rev., 2010, 39, 1478 RSC; (b) G. Magyarfalvi, G. Traczy and E. Vass, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 403 CrossRef CAS.
- (a) P. J. Stephens, F. J. Devlin and J. J. Pan, Chirality, 2008, 20, 643 CrossRef CAS PubMed; (b) P. J. Stephens and M. Lowe, Annu. Rev. Phys. Chem., 1985, 36, 213 CrossRef CAS; (c) H. Sato and A. Yamagishi, Int. J. Mol. Sci., 2013, 14, 964 CrossRef CAS PubMed.
- S. R. Domingos, A. Huerta-Viga, L. Baij, S. Amirjalayer, D. A. Dunnebier, A. J. Walters, M. Finger, L. A. Nafie, B. de Bruin, W. J. Buma and S. Woutersen, J. Am. Chem. Soc., 2014, 136, 3530 CrossRef CAS PubMed.
- (a) C. Merten and Y. Xu, Angew. Chem., Int. Ed., 2013, 52, 2073 CrossRef CAS PubMed; (b) J. Thomas, O. Sukhorukov, W. Jäger and Y. Xu, Angew. Chem., Int. Ed., 2014, 53, 1156 CrossRef CAS PubMed.
- (a) Z.-Y. Li, H.-Q. Huang, L. Xu, R.-B. Liu, J.-J. Zhang, S.-Q. Liu and C.-Y. Duan, Cryst. Growth Des., 2013, 13, 918 CrossRef CAS; (b) C. Merten, R. McDonald and Y. Xu, Inorg. Chem., 2014, 53, 3177 CrossRef CAS PubMed; (c) W.-G. Lu, J.-Z. Gu, L. Jiang, M.-Y. Tan and T.-B. Lu, Cryst. Growth Des., 2007, 8, 192 CrossRef; (d) C. Merten and Y. Xu, Dalton Trans., 2013, 42, 10572 RSC; (e) G. Tian, G. Zhu, X. Yang, Q. Fang, M. Xue, J. Sun, Y. Wei and S. Qiu, Chem. Commun., 2005, 1396 RSC; (f) Z. B. Han, B. Y. Li, J. W. Ji, Y. E. Du, H. Y. An and M. H. Zeng, Dalton Trans., 2011, 40, 9154 RSC; (g) Y. Ma, Z. Han, Y. He and L. Yang, Chem. Commun., 2007, 4107 RSC.
- (a) S. L. Piano, S. D. Pietro and L. D. Bari, Chem. Commun., 2012, 48, 11996 RSC; (b) S. Y. Lin, C. Wang, L. Zhao and J. K. Tang, Chem. – Asian J., 2014, 9, 3558 CrossRef CAS PubMed; (c) H. Miyake, K. Terada and H. Tsukube, Chirality, 2014, 26, 293 CrossRef CAS PubMed; (d) J. J. Luo and L. Xu, Inorg. Chem., 2006, 45, 11030 CrossRef CAS PubMed; (e) H. Sato, D. Shirotani, K. Yamanari and S. Kaizaki, Inorg. Chem., 2010, 49, 356 CrossRef CAS PubMed; (f) Y. Zheng, Y.-Y. Pan, Y.-P. Ren, L.-S. Long, R.-B. Huang and L.-S. Zheng, Chem. Commun., 2014, 50, 14728 RSC.
- T. Taniguchi and K. Monde, J. Am. Chem. Soc., 2012, 134, 3695 CrossRef CAS PubMed.
- A. Castaings, J.-C. Marchon, D. Cavagnat and T. Buffeteau, Chirality, 2013, 25, 480 CrossRef CAS PubMed.
- (a) I. J. Hewitt, J. Tang, N. Madhu, C. E. Anson, Y. Lan, J. Luzon, M. Etienne, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 6352 CrossRef CAS PubMed; (b) P. Zhang, Y. N. Guo and J. K. Tang, Coord. Chem. Rev., 2013, 257, 1728 CrossRef CAS PubMed; (c) L. Sorace, C. Benelli and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3092 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis of 1a and 1b, crystal data, Tables S1–S3, and Fig. S1–S9. CCDC 1029797 and 1029798. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt00404g |
| This journal is © The Royal Society of Chemistry 2015 |