Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
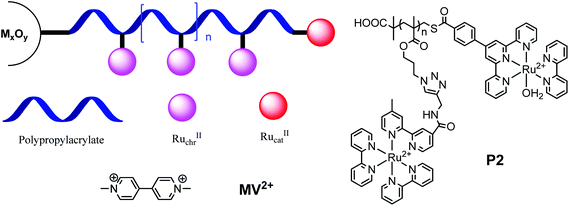
Polypyridyl Ru(II)-derivatized polypropylacrylate polymer with a terminal water oxidation catalyst. Application of reversible addition–fragmentation chain transfer polymerization†
Zhen
Fang
a,
Akitaka
Ito
ab,
Hanlin
Luo
a,
Dennis L.
Ashford
a,
Javier J.
Concepcion
a,
Leila
Alibabaei
a and
Thomas J.
Meyer
*a
aDepartment of Chemistry, University of North Carolina at Chapel Hill, CB3290, Chapel Hill, North Carolina 27599, USA. E-mail: tjmeyer@unc.edu
bDepartment of Chemistry, Graduate School of Science, Osaka City University, 3-3-138, Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan
First published on 25th March 2015
Abstract
A Ru(II) polypyridyl-derivatized polypropylacrylate end-capped with a water-oxidation-catalyst (WOC) has been synthesized by using reversible addition–fragmentation chain transfer polymerization (RAFT) followed by click reaction and end-group functionalization. In cyclic voltammograms in propylene carbonate, chromophore oxidation occurs at 1.27 V vs. NHE and the RuIII/II wave for the catalyst at 0.84 V vs. NHE. Upon excitation of the Ru(II) chromophore, excited-state energy migration occurs by site-to-site, –RuII*– → –RuII–, energy transfer hopping along the polymer chain, in part, reaching the terminal catalyst site where –RuII*– → –RuII–OH22+ energy transfer is favored by ΔGen = −2100 cm−1. Added MV2+ as an electron transfer acceptor oxidizes the –RuII*– excited state on the polymer to Ru(III), –RuII*– + MV2+ → –RuIII– + MV+, and ultimately, the catalyst, by site-to-site electron transfer hopping and oxidation, 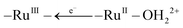 . Oxidation is followed by relatively slow, diffusional back electron transfer from MV˙+ to Ru(III) sites on the polymer chain. The mixed chromophore-catalyst polymer is a water oxidation catalyst with potential for enhanced light harvesting and water oxidation.
. Oxidation is followed by relatively slow, diffusional back electron transfer from MV˙+ to Ru(III) sites on the polymer chain. The mixed chromophore-catalyst polymer is a water oxidation catalyst with potential for enhanced light harvesting and water oxidation.
Introduction
Dye-sensitized photoelectrosynthesis cells (DSPEC) provide a straightforward design for solar conversion to fuels through water splitting to hydrogen and oxygen, and/or CO2 reduction to reduced carbon fuels.1 Water oxidation catalysis is a key element in such designs with stepwise oxidative activation occurring on the surfaces of high band gap semiconductor oxides.2 Given the wide band gap of useful semiconductors such as TiO2, with a band gap of 3.2 eV, an integrated chromophore sensitizer is required to absorb light, undergo excited-state injection, and transfer oxidizing equivalents to an associated catalyst for water oxidation. Considerable progress has been made in designing and investigating appropriate chromophore-catalyst assemblies.3One successful strategy is surface binding of assemblies4 to the surface of TiO2.5 Excitation of the chromophore and electron injection by the resulting chromophore excited state into the TiO2 conduction band is followed by oxidative activation of the catalyst by intra-assembly electron transfer. In a recent example, use of a conjugated bridging ligand resulted in trapping of the excited-state electrons at the lowest-lying metal-to-ligand charge transfer (MLCT) state localized on the ligand, greatly decreasing the electron injection efficiency.6 In a modification, chromophore-catalyst assemblies with non-conjugated bridging ligands were used to significantly increase injection,7 providing a feasible assembly design strategy for DSPEC applications.
Another strategy is use of multiple chromophores in the chromophore-catalyst assembly as a way of enhancing the effective solar insolation rate which in ambient sunlight is 1–2 s−1.8 Ru(II) polypyridyl complexes have been incorporated into polymer scaffolds and utilized with the scaffolds used as light-harvesting antenna with high optical cross sections,9 with rapid, efficient charge/exciton transport between the adjacent ruthenium pendant units.10 In this strategy, excitation of a single chromophore in the polymer chain,  , is followed by energy migration between adjacent units, –RuII*–RuII– → –RuII–RuII*–, ultimately with electron injection into the semiconductor, (TiO2–RuII*– → TiO2(e−)–RuIII–). Following injection, electron-transfer migration along the polymer strand occurs with oxidation of the catalyst, –RuIII–RuIIcat → –RuII–RuIIIcat–, beginning the 4e−/4H+ sequence for water oxidation.
, is followed by energy migration between adjacent units, –RuII*–RuII– → –RuII–RuII*–, ultimately with electron injection into the semiconductor, (TiO2–RuII*– → TiO2(e−)–RuIII–). Following injection, electron-transfer migration along the polymer strand occurs with oxidation of the catalyst, –RuIII–RuIIcat → –RuII–RuIIIcat–, beginning the 4e−/4H+ sequence for water oxidation.
We report here the synthesis of a poly(propylmethacrylate) derivative (P2, Chart 1) end-capped with the catalyst derivative, [[4′-phenyl-2,2′:6′,2′′-terpyridine]Ru(bpy)(H2O)]2+ (bpy = 2,2′-bipyridine), by reversible addition–fragmentation chain transfer (RAFT) polymerization followed by a click reaction and end group functionalization. The RAFT polymerization features a dual end group-functionality in a one-pot synthesis and a side-functionality for adding Ru(II) polypyridyl chromophores by click coupling.
Several advantages are featured in this molecular design. The introduction of multiple Ru(II) chromophores enhances the light harvesting ability of the assembly relative to a single chromophore. Multi-site light absorption coupled with rapid intra-strand energy migration results in an increase in the effective rate of solar insolation. In the molecular design developed here, a channel exists for electron transfer hopping to and from the terminal catalyst through the Ru(II) sites on the polymer. Given the saturated spacers between sites on the polymer strands, inter-site electron transfer is presumably dominated by outer-sphere contact and orbital overlap. Because the catalyst site is at a terminus, it is held at a long distance from the oxide surface creating a spatial barrier to back electron transfer from the metal oxide surface to the catalyst.11
With these advantages in mind, we report here the synthesis and initial photophysical and electrochemical properties of a first example of a catalyst-terminated, polymeric Ru(II) polypyridyl assembly.
Results and discussion
Scheme 1 illustrates the synthetic route to the catalyst terminated, poly-chromophoric polymer, P2, with details provided in ESI.† 3-Chloropropyl methacrylate (1) was synthesized by condensation of methacryloyl chloride with 3-chloropropan-1-ol in the presence of triethylamine as the catalyst. Bulk polymerization of the acrylate yielded the polymer precursor PCPM in the presence of AIBN as the catalyst and 2-(dodecylthiocarbonothioylthio)-2-methylpropanoic acid (DDMAT, prepared by using a literature method12) as the RAFT agent. The chloride group in PCPM was substituted with azide upon treatment with NaN3, monitored by loss of a resonance at 3.65 ppm and appearance of a new resonance at 3.42 ppm in 1H-NMR spectra, Fig. S1 in the ESI.† The conversion yield was close to 96% calculated from the proton resonance integration. The trithiolester group disappeared after NaN3 treatment with a white solid appearing due to the loss of trithiolester group introduced by DDMAT. This is consistent with the absence of a –C12H25 proton resonance (∼1.25 ppm) in the trithiolester group in the PNPM1H_NMR spectrum. The removal of trithiolester under basic condition has been reported in previous research.9 GPC analysis shows that the number average molecular weight (Mn) of PCPM is 3530 g mol−1 (degree of polymerization: ∼20); while the Mn of PNPM is 3090 g mol−1. The decrease in Mn is in agreement with the cleavage of the trithiol group, while the polydispersity index (PDI) remains constant (PDI ∼ 1.3, see GPC curves in Fig. S2 in the ESI†).The precursor polymer P1 was obtained by grafting [(4-CC-bpy)Ru(bpy)2]2+ onto the PNPM side chains via a click reaction between the azidopropyl functionality and the alkyne unit of [(4-CC-bpy)Ru(bpy)2]2+. The orange precipitate from ethanol yielded a metallopolymer with PF6− as the counter ion, which is soluble in acetone, acetonitrile and DMF. Counter-ion metathesis to chloride provides the basis for further purification by dialysis (cutoff Mn ∼ 3500 Dalton) to remove unreacted [(4-CC-bpy)Ru(bpy)2]2+. In the infrared spectrum of P1 there is no azide stretch at ∼2100 cm−1 consistent with the click reaction being essentially quantitative (See Fig. S3 in the ESI†). The 1H_NMR spectrum of P1 was dominated by resonances of the bipyridine ligands in the aromatic region. P1 was functionalized by condensation between the –SH terminus and [[4-([2,2′:6′,2′′-terpyridin]-4′-yl)benzoyl chloride]Ru(bpy)(Cl)]+, which was synthesized based on a previously described method.13 Dialysis was conducted to remove excess [[4-([2,2′:6′,2′′-terpyridin]-4′-yl)benzoyl chloride]Ru(bpy)(Cl)]+, which was hydrolyzed to [[4-([2,2′:6′,2′′-terpyridin]-4′-yl)benzoyl acid]Ru(bpy)(Cl)]+ during work-up. Treatment with silver triflate (AgOTf) resulted in the end group Ru(II) catalyst unit coordinated with OTf−. Precipitation from saturated LiClO4 produced a polymer with RuIIcatin situ coordinated with H2O and counter ion metathesis from OTf− to ClO4−. The 1H_NMR spectrum of P2 shows a pattern similar to P1, with the exception of a growing overlapped resonance at ∼7.2 ppm and a weak resonance at ∼9.5 ppm attributed to the protons at the terpyridine and bipyridine ligands of the catalyst.
Electrochemistry
The electrochemical properties of P1 and P2 were studied by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in both solution and on nano-ITO (tin-doped indium oxide) substrates. Fig. 1 shows anodic DPV scan for P1, P2, and a mixture of P1 with [[4′-phenyl-2,2′:6′,2′′-terpyridine]Ru(bpy)(H2O)]2+ (RuIIcat–H2O), where the percentage of P1 repeat units relative to [[4′-phenyl-2,2′:6′,2′′-terpyridine]Ru(bpy)(H2O)]2+ (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was identical to the ratio of Ru chromophore to terminal units in P2 (20
1) was identical to the ratio of Ru chromophore to terminal units in P2 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The DPV scan for P1 features a typical RuIII/RuII oxidation at 1.27 V vs. NHE. The mixture of P1 and [[4′-phenyl-2,2′:6′,2′′-terpyridine]Ru(bpy)(H2O)]2+ exhibits a wave at 0.82 V vs. NHE for the RuIIIcat–H2O/RuIIcat–H2O redox couple and a barely discernible feature at 1.04 V vs. NHE attributed to the PCET inhibited RuIVcat
1). The DPV scan for P1 features a typical RuIII/RuII oxidation at 1.27 V vs. NHE. The mixture of P1 and [[4′-phenyl-2,2′:6′,2′′-terpyridine]Ru(bpy)(H2O)]2+ exhibits a wave at 0.82 V vs. NHE for the RuIIIcat–H2O/RuIIcat–H2O redox couple and a barely discernible feature at 1.04 V vs. NHE attributed to the PCET inhibited RuIVcat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/RuIIIcat–H2O couple. The RuIVcat
O/RuIIIcat–H2O couple. The RuIVcat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/RuIIIcat–H2O wave is kinetically distorted because of the proton-coupled electron transfer (PCET) nature of the half reaction arising from formation of Rucat
O/RuIIIcat–H2O wave is kinetically distorted because of the proton-coupled electron transfer (PCET) nature of the half reaction arising from formation of Rucat![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O14 in the propylene carbonate medium. For P2 only the RuIIIcat–H2O/RuIIcat–H2O oxidation was observed, at E1/2 = 0.84 V vs. NHE. As expected given the chromophore
O14 in the propylene carbonate medium. For P2 only the RuIIIcat–H2O/RuIIcat–H2O oxidation was observed, at E1/2 = 0.84 V vs. NHE. As expected given the chromophore![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio in the assembly, the peak current ratio for chromophore to catalyst was close to the 1
catalyst ratio in the assembly, the peak current ratio for chromophore to catalyst was close to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio expected given the composition of the assembly.15 Similar results were obtained for the polymer assembly on nano-ITO electrodes (see Fig. S4 in the ESI†).
20 ratio expected given the composition of the assembly.15 Similar results were obtained for the polymer assembly on nano-ITO electrodes (see Fig. S4 in the ESI†).
Absorption and emission spectra
In solution, UV-visible spectra of P1 and P2 share similar absorption features with a small contribution from –RuIIcat–OH2 relative to the Ru(II) chromophores in P2, Fig. 2a. The metal-to-ligand charge transfer (MLCT) absorption manifolds maximize at 460 nm, with π–π* bpy-based absorptions at 290 nm. In the spectrum of P2 a slight redshift in the π–π* absorption is observed with a slight broadening for the MLCT manifold similar to the sum of the spectra of P1 and RuIIcat–H2O. Similar observations were made in the emission spectra in Fig. 2b with the observation of ∼15% emission quenching for P2 compared to P1. For the RuIIcat–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture with P1 control, less than 2% quenching occurs. The results are consistent with excitation and energy transfer migration within the polymer strand for P2 to the lower-lying RuIIcat–H2O-based excited state which is a weak emitter with a short-lived excited state.
20) mixture with P1 control, less than 2% quenching occurs. The results are consistent with excitation and energy transfer migration within the polymer strand for P2 to the lower-lying RuIIcat–H2O-based excited state which is a weak emitter with a short-lived excited state.
 | ||
Fig. 2 (a) Absorption and (b) emission spectra of P1, P2, RuIIcat–H2O ([[4′-phenyl-2,2′:6′,2′′-terpyridine]Ru(bpy)(H2O)]2+) and a mixture of P1 with RuIIcat–H2O, (c) wavenumber-scale emission spectra of P1, P2 and RuIIcat–H2O at 298 K with simulated spectra (olive curves) obtained by single-mode Franck–Condon analysis of emission spectra (eqn (1)) with the fitting parameters listed in Table 1, and (d) emission decay profiles for P1, P2 and the mixture of P1 and RuIIcat–H2O in DMF at 298 K monitored at 650 nm. Ru(II) concentration, 10 μM; ratio of Ru(II) in P1 to RuIIcat–H2O in the mixed sample was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; excitation wavelength, 445 nm. 1; excitation wavelength, 445 nm. | ||
Emission spectra were analyzed by application of a single mode, Franck–Condon analysis as described previously.16 Observed and simulated spectra are shown in Fig. 2c. The spectra are broad and featureless, characteristic of MLCT spectra of Ru(II) polypyridyl complexes at room temperature.17
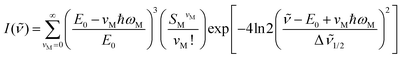 | (1) |
In eqn (1), I(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) is the emission intensity at the energy
) is the emission intensity at the energy ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) in wavenumbers (cm−1), relative to the intensity of the 0→0 transition. E0 is the energy gap between the zero vibrational levels of the ground and excited states. vM, ħωM and SM are the vibrational quantum number, the quantum spacing and the Huang–Rhys factor reflecting the degree of distortion in the single, average mode as the difference in equilibrium displacements, respectively. Δ
in wavenumbers (cm−1), relative to the intensity of the 0→0 transition. E0 is the energy gap between the zero vibrational levels of the ground and excited states. vM, ħωM and SM are the vibrational quantum number, the quantum spacing and the Huang–Rhys factor reflecting the degree of distortion in the single, average mode as the difference in equilibrium displacements, respectively. Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 1/2 is the full width at half-maximum (fwhm) for individual vibronic lines. Emission intensities, corrected to wavenumbers by I(
1/2 is the full width at half-maximum (fwhm) for individual vibronic lines. Emission intensities, corrected to wavenumbers by I(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) = [I(λ)]λ2, were fit by optimizing the parameters E0, Δ
) = [I(λ)]λ2, were fit by optimizing the parameters E0, Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 1/2, ħωM, and SM with a least squares minimization routine which utilizes a generalized reduced gradient (GRG2) algorithm.18 The summation was carried out over 11 ground-state vibrational levels (vM = 0 → 10). The free energy content of the polymer-based excited state above the ground state, ΔGES, was calculated by eqn (2) with values presented in Table 1.
1/2, ħωM, and SM with a least squares minimization routine which utilizes a generalized reduced gradient (GRG2) algorithm.18 The summation was carried out over 11 ground-state vibrational levels (vM = 0 → 10). The free energy content of the polymer-based excited state above the ground state, ΔGES, was calculated by eqn (2) with values presented in Table 1.
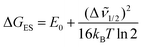 | (2) |
| Sample | E 0/cm−1 | Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 1/2/cm−1 1/2/cm−1 |
ħω M/cm−1 | S M | r | λ o/cm−1 | ΔGES/cm−1 |
|---|---|---|---|---|---|---|---|
| a Correlation coefficient. | |||||||
| P1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
1900 | 1350 | 0.86 | 0.99988 | 1570 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (2.13 eV) 200 (2.13 eV) |
| P2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 590 |
1930 | 1380 | 0.85 | 0.99986 | 1620 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 (2.13 eV) 210 (2.13 eV) |
| RuIIcat–H 2 O | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1800 | 1330 | 0.68 | 0.99936 | 1400 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 (1.87 eV) 060 (1.87 eV) |
Table 1 summarizes spectral fitting results for both polymers and catalyst RuIIcat–H2O. The catalyst emits at lower energy and is short-lived compared to the chromophore sites in P1. The decrease in lifetime for P2 is consistent with intra-strand energy transfer to the catalyst by site-to-site hopping followed by energy transfer to the catalyst and its rapid decay. In eqn (2), kB and T are the Boltzmann constant and absolute temperature, respectively. The free energy change for energy transfer from chromophore excited state to catalyst, ΔGen, was calculated by eqn (3) with (acceptor) the catalyst energy acceptor and (donor) the excited polymer chromophore.
| ΔGen = ΔGES (acceptor) − ΔGES (donor) | (3) |
Based on the values in Table 1, the free energy change for –RuII*– to RuIIcat–H2O energy transfer is favorable with ΔGen ∼ −2100 cm−1 (−0.26 eV) showing that emission quenching in P2 relative to P1 is consistent with intrastrand energy transfer to the terminal catalyst site.
Emission decay
Emission decays were obtained by time-correlated single photon counting (TCSPC) with the results illustrated in Fig. 2d. All emission decays were multiple exponential consistent with heterogeneity and a distribution of emitting sites along the polymer chain. Both P1 (∼840 ns) and the sample mixture (∼800 ns) have comparable average lifetimes with the lifetime of P2 decreased to ∼640 ns consistent with partial intra-strand quenching.Excited-state quenching
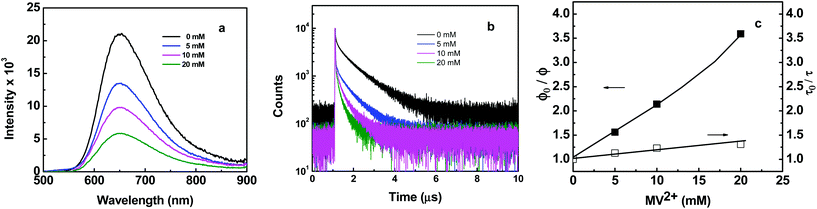
The dynamics of excited state electron transfer quenching with added electron-transfer quencher methylviologen dication (MV2+) were investigated by time-resolved fluorescence and transient absorption measurements in DMF at room temperature. In these experiments, the concentration of MV2+ was varied from 0–20 mM.Fig. 3a and b show emission spectra and emission decay profiles for P2 in the absence and presence of MV2+. With the addition of MV2+, both rapid and slow quenching components are observed (Fig. 3b). The rapid component is due to pre-association of the quencher with Ru(II) sites along the polymer chains.19 Absorption or emission-time dynamics for the slow component are bi-exponential. Fig. 3c shows Stern–Volmer plots for P2 quenching by both quenching pathways. The slow data follow Stern–Volmer kinetics with, τ0/τ = 1 + kq,diffτ0[MV2+]. In this expression, τ0 is the emission lifetime of P2 (∼800 ns) in the absence of MV2+, and τ in the presence of MV2+. kq,diff is the rate constant for the diffusional quenching component with kq,diff ∼ 2.0 × 107 M−1 s−1 from the slope of the Stern–Volmer plot.
The kinetics for the rapid quenching component are complicated by overlapping contributions from pre-associated quencher and slower diffusional quenching. Emission quenching data were treated by use of the expression, ϕ0/ϕ = (1 + Ks [MV2+])(1 + kq,diffτ0[MV2+]) = 1 + (kq,diff + kq)τ0 [MV2+] + kqkq,diffτ02[MV2+]2. In this expression, Ks is the association constant between quencher and polymer and kq is the quenching rate constant for the pre-associated quencher-polymer complex. Based on the data for the rapid and slow quenching components in Fig. 3c, kq ∼ 1.04 × 108 M−1 s−1.
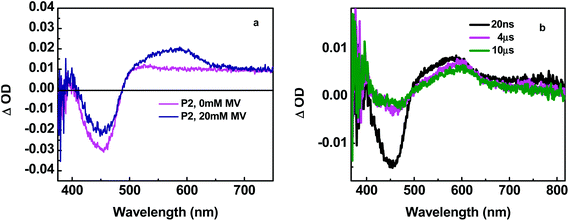
In transient absorption spectra, addition of MV2+ and excited-state quenching of –RuII*– by oxidative electron transfer gives rise to new absorption features at 396 and 607 nm,20,21Fig. 4 and S6.† These features are characteristic of the reduced radical cation, MV˙+, formed in the reaction, –RuII*– + MV2+ → –RuIII– + MV+. The formal potential for the –RuIII–/–RuII*– couple is Eox(III/II*) = −0.86 V vs. NHE as calculated from, Eox(III/II*) = E(III/II) − ΔGES/nF. In this relationship F is the Faraday constant in eV per equivalent, with E(III/II) = 1.27 V vs. NHE from the electrochemical measurements and ΔGES = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1 (2.13 eV) from emission spectral fitting. With E(MV2+/MV˙+) = −0.45 V vs. NHE,22 the oxidation of –RuII*– by MV2+ is thermodynamically favored with ΔG°′ = ∼0.4 eV.
200 cm−1 (2.13 eV) from emission spectral fitting. With E(MV2+/MV˙+) = −0.45 V vs. NHE,22 the oxidation of –RuII*– by MV2+ is thermodynamically favored with ΔG°′ = ∼0.4 eV.
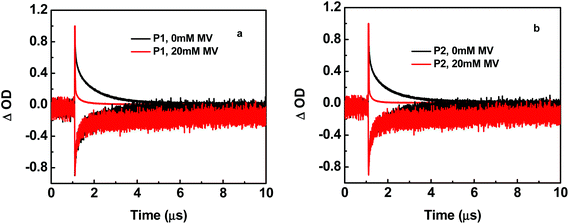
As shown in Fig. 4, the transient absorption difference spectrum for P2, following 425 nm laser flash excitation, obtained at 20 ns after the laser flash, exhibits features similar to those for P1 (Fig. S5a in the ESI†). In these spectra, a π–π* absorption feature for a reduced ligand appears at ∼390 nm and a bleach for the loss of the MLCT absorption feature at ∼460 nm.23 Both emission decays at 630 nm for P1 and P2 in the absence of MV2+ were non-exponential but could be fit to multi-exponential kinetics with contributions to the complexity from excited state intra-strand energy transfer hopping and inhomogenities at the individual sites in the polymers.8,9 Note the quenching and back electron transfer scheme in Fig. 6. The data were fit to a biexponential function with a rapid decay component with τ = 20–30 ns and a slower decay component with τ ∼ 1 μs. The kinetics of loss of the bleach at 460 nm for both polymers exhibited kinetics that mirrored the 630 nm emission decay (Fig. 5).
A contribution to the slow, 10 μs, growth at 460 nm appears due to relatively slow back electron transfer from MV˙+ to Ru(III) (Fig. 4 and S5 in ESI,† which occurs in concert with the decrease in absorbance by MV˙+ at 600 nm. Following excitation, the excited chromophore, –RuII*–, is oxidized by pre-associated or diffusional MV2+. Following oxidation, –RuIII– undergoes electron transfer with adjacent sites along the polymer chain resulting in net hole migration. Intra-strand migration eventually reaches the low potential catalyst site at the terminus, –RuIIIcat, on the ∼ns time range in competition with back electron transfer with MV˙+, Fig. 6.
Back electron transfer from MV˙+ to either –RuIIIcat or –RuIIIchr– is highly favorable given the 1.27 V vs. NHE potential for the –RuIII/II– couple. Back electron transfer kinetics were studied by analyzing absorbance-time traces for loss of MV˙+ at 600 nm. As shown in Fig. S7,† the data could be fit to equal concentration, second order kinetics consistent with the reaction, MV˙+ + Ru(III) → MV2+ + Ru(II), according to the expression,  , with [MV˙+] = ΔOD/εl. [MV˙+]o, ε, l and ΔOD are the initial concentration of MV˙+, the molar extinction coefficient for MV˙+ (∼13
, with [MV˙+] = ΔOD/εl. [MV˙+]o, ε, l and ΔOD are the initial concentration of MV˙+, the molar extinction coefficient for MV˙+ (∼13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1), l, the light path, was 1 cm, and ΔOD the optical density change.13 Back electron-transfer rate constants obtained from the data for the reactions between MV˙+ and Ru(III) in P1 and P2 were 7.6 × 109, and 8.2 × 109 M−1 s−1, respectively.
800 M−1 cm−1), l, the light path, was 1 cm, and ΔOD the optical density change.13 Back electron-transfer rate constants obtained from the data for the reactions between MV˙+ and Ru(III) in P1 and P2 were 7.6 × 109, and 8.2 × 109 M−1 s−1, respectively.
Transient absorption and electrochemical measurements on oxide surfaces
Given the ultimate interest in utilizing molecular assemblies in DSPEC applications, the photophysical and electrochemical properties of polymers on the surfaces of nanoparticle, mesoscopic metal oxide films of ZrO2, TiO2 and ITO were also investigated. Nano-TiO2 films and nano-ZrO2 films, typically 6 μm thick, coating an area, 11 mm × 11 mm, on FTO (fluorine-doped SnO2) glass, were prepared according to previously published procedures.24 Chromophore adsorption in the nanoparticle films was carried out by immersing the resulting slides in 0.2 mM solutions of the polymers in DMF solutions, followed by soaking an additional 4 h in pure DMF solvent to remove any aggregates/free polymers in the film. The slides were removed, rinsed with methanol, and dried under a stream of nitrogen. Surface coverages (Γ in mol cm−2) were estimated by UV-visible measurements by using the expression Γ = A(λ)/ε(λ)/1000, with solution molar extinct coefficients (ε) used in the calculations.25A(λ) was the maximum absorbance of the derivatized slides.Due to the large molecular size of the polymer assemblies, with ∼20 repeat units and molecular weights over 20 K, and only a single anchoring group at the end, low surface coverages (10−10–10−9 mol cm−2 in Ru(II) polypyridyl sites) were obtained on both nanoZrO2, and nanoTiO2. The limited surface coverages suggest that surface binding may be limited, in part due to the limited internal volume of the voids in the oxide films given the large molecular volumes of the polymers. We recently reported that within the TiO2 nanoparticle pores (∼20 nm), the maximum number of Ru(II) polypyridyl complexes that could be grown from a surface-bound precursor was seven.26 The polymer-catalyst assemblies reported here with ∼20 Ru(II) bpy units is too large to enter the cavities in the films resulting in low coverages. The polymer presumably occupies channels and the surfaces of the oxide films.
For P2 compared to P1 on ZrO2 nanoparticle films, partial quenching was observed with propylene carbonate as the external solvent, Fig. S8.† The conduction band potential for ZrO2 at −1.4 V vs. NHE at pH 7 in water, is inaccessible for injection by the polymer-based –RuII*– excited state with Eox(III/II*) = −0.86 V.27 The decreased emission intensity for P2 relative to P1, as in solution, is presumably due to intra-strand energy transfer migration to the terminal catalyst site which acts as an energy transfer trap.
With TiO2 as the substrate, substantial emission quenching was observed for both P1 and P2, due to the excited state electron injection into TiO2. Injection efficiencies for both polymers on TiO2 are comparable, ∼50% as estimated from the decrease in emission intensities, Fig. S8.†
Electrocatalytic water oxidation by P2 was investigated on ∼6 μm nanoparticle, mesoscopic films of nanoITO. As estimated by UV-visible measurements, the surface coverage was ∼1.6 × 10−10 mol cm−2. The derivatized oxide was used as the working electrode in a three compartment electrochemical cell with a Pt wire as the counter electrode and AgNO3/Ag as the reference in propylene carbonate 0.1 M in Bu4NPF6. Cyclic voltammograms are shown in Fig. 7 relative to the un-derivatized electrode as a background.
With increasing amounts of added water, from 0% to 8% by volume, the Ru(III)/Ru(II) couple shifts from 1.29 V to 1.20 V due to a selective outer sphere solvation effect. A noticeable increase in current at ∼1.8 V with successive additions of water also appears in the voltammograms. The enhanced current for the assembly-derivatized electrode relative to ITO and P1/ITO is notable and consistent with water oxidation catalysis by the terminal single site catalyst28 Possible photochemically driven water oxidation catalysis is currently under investigation.
Conclusions
We have described here the preparation and properties of a Ru(II) polypyridyl-derivatized polypropylacrylate polymer with a water oxidation catalyst at the terminus. The polymer was synthesized by RAFT polymerization followed by click reaction coupling to the polymer backbone and then end-group functionalization. In the final chromophore-catalyst assembly polymer, the chromophore![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio was 20
catalyst ratio was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The redox properties of the terminal catalyst are observed in both cyclic and differential pulse voltammograms. Upon excitation of the chromophore-catalyst polymer there is evidence for excitation of the chromophore on the polymer backbone followed by energy transfer migration and quenching by energy transfer to the catalyst which is favored by ΔGen = −2100 cm−1 (−0.26 eV). With added MV2+, electron transfer quenching of the MLCT excited states of the Ru(II) chromophores on the polymer occurs, as shown by transient absorption measurements and the appearance of MV˙+. Electron transfer quenching is followed by site-to-site hole migration along the polymer chain to the terminal catalyst site in competition with back electron transfer from MV˙+.
1. The redox properties of the terminal catalyst are observed in both cyclic and differential pulse voltammograms. Upon excitation of the chromophore-catalyst polymer there is evidence for excitation of the chromophore on the polymer backbone followed by energy transfer migration and quenching by energy transfer to the catalyst which is favored by ΔGen = −2100 cm−1 (−0.26 eV). With added MV2+, electron transfer quenching of the MLCT excited states of the Ru(II) chromophores on the polymer occurs, as shown by transient absorption measurements and the appearance of MV˙+. Electron transfer quenching is followed by site-to-site hole migration along the polymer chain to the terminal catalyst site in competition with back electron transfer from MV˙+.
Electron transfer and excited state properties are retained by surface binding of the catalyst-derivatized polymer on the surfaces of nanoparticle, mesoscopic oxide electrodes as shown by electrochemical and transient spectroscopic measurements. The polymer chromophore-terminal catalyst assembly is important in demonstrating a new chromophore-quencher design. It introduces multiple light absorbers in a polymeric framework for enhanced light harvesting with a terminal water oxidation catalyst. The polymer is end-group derivatized for surface binding to oxide substrates.
Future work will focus on improvement of assembly design to enhance surface loading and possible applications in DSPEC devices for water splitting. In the next generation of polymer assembly, more efficient water oxidation catalysts based on carbene and Mebimpy ligands,28,29 will be introduced.
Acknowledgements
This material is based upon work solely supported as part of the UNC EFRC: Center for Solar Fuels, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Science under Award Number DE-SC0001011.Notes and references
- (a) W. J. Youngblood, S.-H. Lee, Y. Kobayashi, E. A. Hernandez-Pagan, P. G. Hoertz, T. A. Moore, A. L. Moore, D. Gust and T. E. Mallouk, J. Am. Chem. Soc., 2009, 131, 926 CrossRef CAS PubMed; (b) D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2009, 42, 1890 CrossRef CAS PubMed; (c) L. Sun, L. Hammarstrom, B. Akermark and S. Styring, Chem. Soc. Rev., 2001, 30, 36 RSC; (d) W. Song, Z. Chen, M. K. Brennaman, J. Concepcion and T. J. Meyer, Pure Appl. Chem., 2011, 83, 749 CrossRef CAS; (e) B. Zhang, F. Li, F. Yu, X. Wang, X. Zhou, H. Li, Y. Jiang and L. Sun, ACS Catal., 2014, 4, 804 CrossRef CAS.
- (a) W. J. Youngblood, S.-H. Lee, K. Maeda and T. E. Mallouk, Acc. Chem. Res., 2009, 42, 1966 CrossRef CAS PubMed; (b) M. Yagi and M. Kaneko, Chem. Rev., 2001, 101, 21 CrossRef CAS PubMed; (c) Y. Zhao, J. R. Swierk, J. D. Megiatto Jr., B. Sherman, W. J. Youngblood, D. Qin, D. M. Lentz, A. L. Moore, T. A. Moore, D. Gust and T. E. Mallouk, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15612 CrossRef CAS PubMed; (d) L. Alibabaei, H. Luo, R. L. House, P. G. Hoertz, R. Lopez and T. J. Meyer, J. Mater. Chem. A, 2013, 1, 4133 RSC.
- (a) T. Meyer, J. Papanikolas and C. Heyer, Catal. Lett., 2011, 141, 1 CrossRef CAS PubMed; (b) L. Alibabaei, M. K. Brennaman, M. R. Norris, B. Kalanyan, W. Song, M. D. Losego, J. J. Concepcion, R. A. Binstead, G. N. Parsons and T. J. Meyer, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 20008 CrossRef CAS PubMed; (c) W. R. McNamara, R. L. Milot, H. Song, R. C. Snoeberger III, V. S. Batista, C. A. Schmuttenmaer, G. W. Brudvig and R. H. Crabtree, Energy Environ. Sci., 2010, 3, 917 RSC.
- (a) C. Herrero, A. Quaranta, R.-A. Fallahpour, W. Leibl and A. Aukauloo, J. Phys. Chem. C, 2013, 117, 9605–9612 CrossRef CAS; (b) J. A. Stull, R. D. Britt, J. L. McHale, F. J. Knorr, S. V. Lymar and J. K. Hurst, J. Am. Chem. Soc., 2012, 134, 19973 CrossRef CAS PubMed; (c) J. A. Treadway, J. A. Moss and T. J. Meyer, Inorg. Chem., 1999, 38, 4386 CrossRef CAS.
- D. L. Ashford, W. Song, J. J. Concepcion, C. R. K. Glasson, M. K. Brennaman, M. R. Norris, Z. Fang, J. L. Templeton and T. J. Meyer, J. Am. Chem. Soc., 2012, 134, 19189 CrossRef CAS PubMed.
- W. Song, C. R. K. Glasson, H. Luo, K. Hanson, M. K. Brennaman, J. J. Concepcion and T. J. Meyer, J. Phys. Chem. Lett., 2011, 2, 1808–1813 CrossRef CAS.
- M. R. Norris, J. J. Concepcion, D. P. Harrison, R. A. Binstead, D. L. Ashford, Z. Fang, J. L. Templeton and T. J. Meyer, J. Am. Chem. Soc., 2013, 135, 2080 CrossRef CAS PubMed.
- Y. Tamaki, T. Morimoto, K. Koike and O. Ishitani, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15678 CrossRef PubMed.
- Y. Sun, Z. Chen, E. Puodziukynaite, D. M. Jenkins, J. R. Reynolds and K. S. Schanze, Macromolecules, 2012, 45, 2632 CrossRef CAS.
- (a) L. M. Dupray, M. Devenney, D. R. Striplin and T. J. Meyer, J. Am. Chem. Soc., 1997, 119, 10243 CrossRef CAS; (b) C. N. Fleming, K. A. Maxwell, J. M. DeSimone, T. J. Meyer and J. M. Papanikolas, J. Am. Chem. Soc., 2001, 123, 10336 CrossRef CAS PubMed.
- M. K. Brennaman, A. O. T. Patrocinio, W. Song, J. W. Jurss, J. J. Concepcion, P. G. Hoertz, M. C. Traub, N. Y. Murakami Iha and T. J. Meyer, ChemSusChem, 2011, 4, 216 CrossRef CAS PubMed.
- Z. Liu, J. Hu, J. Sun and G. Liu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4922 CrossRef CAS PubMed.
- D. L. Ashford, D. J. Stewart, C. R. Glasson, R. A. Binstead, D. P. Harrison, M. R. Norris, J. J. Concepcion, Z. Fang, J. L. Templeton and T. J. Meyer, Inorg. Chem., 2012, 51, 6428 CrossRef CAS PubMed.
- V. Y. Shafirovich, S. H. Courtney, N. Q. Ya and N. E. Geacintov, J. Am. Chem. Soc., 1995, 117, 4920 CrossRef CAS.
- Z. Chen, J. J. Concepcion, H. Luo, J. F. Hull, A. Paul and T. J. Meyer, J. Am. Chem. Soc., 2010, 132, 17670 CrossRef CAS PubMed.
- Z. Murtaza, D. K. Graff, A. P. Zipp, L. A. Worl, W. E. Jones, W. D. Bates and T. J. Meyer, J. Phys. Chem., 1994, 98, 10504 CrossRef CAS.
- A. Ito and T. J. Meyer, Phys. Chem. Chem. Phys., 2012, 14, 13731 RSC.
- D. Fylstra, L. Lasdon, J. Watson and A. Waren, Interfaces, 1998, 28, 29 CrossRef.
- A. Ito, D. J. Stewart, Z. Fang, M. K. Brennaman and T. J. Meyer, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15132 CrossRef CAS PubMed.
- T. Watanabe and K. Honda, J. Phys. Chem., 1982, 86, 2617 CrossRef CAS.
- J. F. Stargardt and F. M. Hawkridge, Anal. Chim. Acta, 1983, 146, 1 CrossRef CAS.
- P. Wardman, J. Phys. Chem. Ref. Data, 1989, 18, 1637 CrossRef CAS PubMed.
- J. H. Alstrum-Acevedo, M. K. Brennaman and T. J. Meyer, Inorg. Chem., 2005, 44, 6802 CrossRef CAS PubMed.
- S. Ito, T. N. Murakami, P. Comte, P. Liska, C. Grätzel, M. K. Nazeeruddin and M. Grätzel, Thin Solid Films, 2008, 516, 4613 CrossRef CAS PubMed.
- L. A. Gallagher, S. A. Serron, X. Wen, B. J. Hornstein, D. M. Dattelbaum, J. R. Schoonover and T. J. Meyer, Inorg. Chem., 2005, 44, 2089 CrossRef CAS PubMed.
- Z. Fang, S. Keinan, L. Alibabaei, H. Luo, A. Ito and T. J. Meyer, Angew. Chem., Int. Ed., 2014, 53, 4872 CrossRef CAS PubMed.
- R. Katoh, A. Furube, T. Yoshihara, K. Hara, G. Fujihashi, S. Takano, S. Murata, H. Arakawa and M. Tachiya, J. Phys. Chem. B, 2004, 108, 4818 CrossRef CAS.
- Z. Chen, J. J. Concepcion, H. Luo, J. F. Hull, A. Paul and T. J. Meyer, J. Am. Chem. Soc., 2010, 132, 17670 CrossRef CAS PubMed.
- M. R. Norris, J. J. Concepcion, Z. Fang, J. Templeton and T. J. Meyer, Angew. Chem., Int. Ed., 2013, 52, 13580 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization details, absorption and emission spectra, GPC, and electrochemistry. See DOI: 10.1039/c5dt00287g |
| This journal is © The Royal Society of Chemistry 2015 |