Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Crystal and magnetic structures of the brownmillerite Ca2Cr2O5
Angel M.
Arevalo-Lopez
and
J. Paul
Attfield
*
Centre for Science at Extreme Conditions (CSEC) and School of Chemistry, University of Edinburgh, Edinburgh, EH9 3FD, UK. E-mail: j.p.attfield@ed.ac.uk
First published on 19th January 2015
Abstract
Powder neutron diffraction and magnetic susceptibility measurements at 10–300 K have been used to determine the crystal and magnetic structures of brownmillerite type Ca2Cr2O5, which was obtained by reduction of the high pressure phase CaCrO3 through hard–soft chemistry. The ambient temperature crystal structure of Ca2Cr2O5 is refined in space group I2mb and the unusual tetrahedral coordination of Cr3+ results in local structural distortions. Cr3+ spins order antiferromagnetically below 220 K and a substantial observed canting of moments shows that Heisenberg exchange is weak or frustrated and competes with antisymmetric Dzialoshinskii–Moriya interactions.
Introduction
Metastable, perovskite-related oxides of transition metals in unusual oxidation states or coordination geometries offer new materials that may have useful magnetic or electronic properties.1 We have recently used ‘hard–soft’ chemistry, in which the instability of a dense precursor prepared under ‘hard’ high pressure-temperature conditions is partially relieved through ‘soft’ post-synthesis modification, to synthesise new vacancy-ordered perovskite oxides.Low-temperature hydrogen reductions of the cubic perovskite SrCrO3, which requires synthesis pressures in excess of 4 GPa,2,3 gave two new SrCrO3−δ phases with oxygen deficiencies δ = 0.2 and 0.25.4 These have complex superstructures due to oxide loss and reconstruction of widely-spaced cubic-(111) anion planes, which relaxes Cr4+ coordination from the octahedral geometry imposed at high pressure to tetrahedral. The SrCrO2.8 superstructure has subsequently been stabilised at ambient pressure by substitution of Fe for Cr.5 The perovskite SrCrO3 and the reduced SrCrO2.8 structure were also stabilised epitaxially as thin films, with rapid oxygen uptake or loss on cycling between the two phases.6
The hard–soft route was also used to discover three reduced CaCrO3−δ phases.7 The high pressure precursor CaCrO3 has an orthorhombically-distorted perovskite structure.8,9 Reductions using a 90% Ar /10% H2 gas mixture at 400–450 °C revealed that three oxygen-deficient superstructure phases were formed, as shown in Fig. 1. The CaCrO3−δ perovskite superstructures are based on stacking of tetrahedral (T) and octahedral (O) layers, with increasing T/(T + O) ratio δ = 0.33 → 0.4 → 0.5 as reduction proceeds. This structural mechanism had not previously been reported for reduced ternary ABO3−δ perovskites but is found in CaTi1−xFexO3−x where Fe-content determines the T/(T + O) layer ratio.10 Magnetic ordering transitions were observed for the three reduced CaCrO3−δ phases at 150–200 K.7
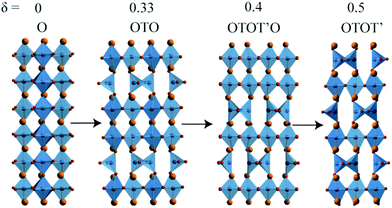 | ||
| Fig. 1 Stacking sequences of octahedral (O) and tetrahedral (T) layers in the CaCrO3−δ series, where the final δ = 0.5 phase is the brownmillerite Ca2Cr2O5. | ||
The most reduced CaCrO3−δ phase, Ca2Cr2O5, has a brownmillerite (Ca2Fe2O5)11 type structure where tetrahedral layers are stacked out-of-phase with their neighbours resulting in the OTOT’ stacking sequence shown in Fig. 1. There is one prior report of a Ca2Cr2O5 brownmillerite prepared at ambient pressure,12 but the structure was not characterized. Ca2Cr2O5 is notable as the brownmillerite structure contains Cr3+ in both octahedral and tetrahedral environments. The latter coordination is rare as crystal field effects provide strong stabilisation of Cr3+ in octahedral environments. The structure of Ca2Cr2O5 was refined in the aristotype Imma brownmillerite structure, in which the chains of tetrahedra in the T layers are disordered, as the X-ray data in the previous study were not sensitive to possible ordered superstructures.7 Here we report a neutron diffraction study of Ca2Cr2O5 which has provided structural characterisation of the chain order, and has also enabled us to solve the low temperature magnetic order of Cr3+ spins.
Experimental
CaCrO3 precursors were synthesized from a stoichiometric mixture of Ca3Cr2O8 and Cr2O3 in a multi-anvil Walker-type press at 9 GPa and 1100° C. A 90% Ar/10% H2 gas mixture was used to reduce CaCrO3 samples to Ca2Cr2O5.Magnetization data were measured on a Quantum Design MPMS SQUID magnetometer. Susceptibilities in zero field cooled (ZFC) and field cooled (FC) conditions were recorded in the 2–300 K temperature range with a 0.5 T applied field.
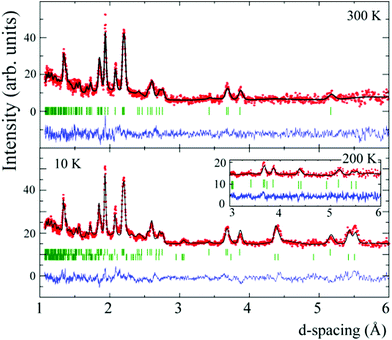
Time-of-flight neutron diffraction data were collected using the GEM diffractometer at the ISIS neutron facility. Several samples were combined in order to obtain a suitable amount for the experiment (∼140 mg). Profiles were recorded at 10, 100, 200 and 300 K. Structural and magnetic models were fitted to data from banks 3, 4 and 5, centred respectively at 2θ = 35, 64 and 91°.
Results and discussion
Crystal structure
No structural phase changes were observed between 10 and 300 K, and the crystal structure of Ca2Cr2O5 was refined using the 300 K neutron data. This structure was previously refined against X-ray data in the aristotype Imma brownmillerite structure, in which the tetrahedral are disordered.7 Ordered tilts of the tetrahedra can give rise to low symmetry superstructures,13 as found in other reported Ca2M2O5 brownmillerites at room temperature. Ca2Fe2O5 has a Pnma superstructure14 while the high pressure phases Ca2Al2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and Ca2Ga2O5
15 and Ca2Ga2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 have polar I2mb (a non-standard setting of Ima2) space group symmetry. A more complex Pcmb supercell with doubling of the c-axis parameter was recently reported in Ca2Co2O5.17 Fits of the two simple ordering models to the 300 K profile of Ca2Cr2O5 gave a slightly better goodness-of-fit χ2 = 1.63 for I2mb than for Pnma where χ2 = 1.69, and no breaking of the body-centring reflection condition was observed. Hence the I2mb model is reported here as the ordered brownmillerite superstructure for Ca2Cr2O5 between 10 and 300 K, and this symmetry is corroborated by the analysis of structural parameters described below. Fits to the profiles are shown in Fig. 2, and refinement results are summarised in Tables 1 and 2.
16 have polar I2mb (a non-standard setting of Ima2) space group symmetry. A more complex Pcmb supercell with doubling of the c-axis parameter was recently reported in Ca2Co2O5.17 Fits of the two simple ordering models to the 300 K profile of Ca2Cr2O5 gave a slightly better goodness-of-fit χ2 = 1.63 for I2mb than for Pnma where χ2 = 1.69, and no breaking of the body-centring reflection condition was observed. Hence the I2mb model is reported here as the ordered brownmillerite superstructure for Ca2Cr2O5 between 10 and 300 K, and this symmetry is corroborated by the analysis of structural parameters described below. Fits to the profiles are shown in Fig. 2, and refinement results are summarised in Tables 1 and 2.
| Atom | Site | x | y | z |
|---|---|---|---|---|
| a Cell parameters a = 5.420(4), b = 14.770(10), and c = 5.509(4) Å; isotropic thermal parameter Uiso = 0.038(2) Å2; residuals Rwp = 0.066 and Rp = 0.052. | ||||
| Ca | 8c | 0.497(3) | 0.1074(4) | 0.012(2) |
| Cr1 | 4a | 0.501(4) | 0.5 | 0.5 |
| Cr2 | 4b | −0.007(4) | 0.25 | 0.936(2) |
| O1 | 8c | 0.754(2) | 0.4919(5) | 0.746(2) |
| O2 | 8c | 0.022(2) | 0.1398(3) | 0.036(1) |
| O3 | 4b | 0.162(2) | 0.75 | 0.366(2) |
| Cr1–O1 × 2 | 1.93(2) | Cr2–O2 × 2 | 1.73(1) |
| Cr1–O1 × 2 | 1.94(2) | Cr2–O3 | 1.83(2) |
| Cr1–O2 × 2 | 2.08(1) | Cr2–O3 | 1.90(2) |
| BVS(Cr1) | 3.02 | BVS(Cr2) | 3.36 |
| O1–Cr1–O1 × 2 | 178.6(12) | O2–Cr2–O2 | 140.9(8) |
| O1–Cr1–O1 × 2 | 88.9(1) | O2–Cr2–O3 × 2 | 99.1(9) |
| O1–Cr1–O1 | 89.8(12) | O2–Cr2–O3 × 2 | 103.7(7) |
| O1–Cr1–O1 | 92.5(12) | O3–Cr3–O3 | 106.9(7) |
| O1–Cr1–O2 × 2 | 87.5(6) | ||
| O1–Cr1–O2 × 2 | 88.2(6) | Cr1–O1–Cr1 | 172.5(5) |
| O1–Cr1–O2 × 2 | 91.7(6) | Cr1–O2–Cr2 | 154.3(6) |
| O1–Cr1–O2 × 2 | 92.6(6) | Cr2–O3–Cr2 | 131.2(10) |
| O2–Cr1–O2 | 173.9(15) | ||
Bond distances and angles for Ca2Cr2O5 in Table 2 allow a detailed analysis of the structural distortions. Bond valence sum (BVS)18 values for the two Cr sites are consistent with the presence of Cr3+, although the value for the Cr2 site is high, as often occurs for strained coordinations. The Cr1O6 octahedron is tetragonally distorted due to the structural connectivity, but O–Cr1–O angles are close to 90 or 180°. However, the Cr2O4 tetrahedron is highly distorted with Cr2–O distances of 1.73–1.90 Å and opening of the O2–Cr2–O2 angle to 141° while other bond angles lie in the range 99–107°. The corresponding GaO4 tetrahedron in isostructural Ca2Ga2O5 is far less distorted, with Ga–O distances of 1.82–1.89 Å and O–Ga–O angles of 106–122°. As Cr3+ and Ga3+ have almost identical ionic radii (0.615 and 0.62 Å for octahedral coordination) this comparison suggests that the excess distortion of Ca2Cr2O5 reflects the instability of tetrahedrally-coordinated Cr3+, as this cation generally has a strong preference for octahedral coordination which maximises crystal field stabilisation energy of the 3d3 electronic configuration. The Ca2+ site coordination is comparable to that in other brownmillerites,14–16 with seven short Ca–O bonds in the range 2.43–2.62 Å while other Ca–O distances are >2.85 Å.
A previously reported study of multiple La1−xAxMnO2.5 (A = Ca, Sr, Ba) brownmillerite compositions showed that the different structural phases fall into distinct regions on a plot of the tetrahedral layer separation (b/2), which has value 7.385 Å for Ca2Cr2O5, against the deviation of the tetrahedral chains from 180°.19 The latter angle is defined as 180°–(O3–O3–O3 angle) and takes value 50.3(7)° in the 300 K structure of Ca2Cr2O5. The point for Ca2Cr2O5 lies within the domain of the I2mb superstructure on the reported structure map, corroborating the space group assignment from the comparison of neutron fits above.
Magnetic structure
Magnetization measurements for the Ca2Cr2O5 sample revealed a Curie tail at low temperatures and a trace of ferromagnetic material, as found in our previous study of CaCrO3−δ phases.7 These impurity contributions were subtracted to yield the susceptibility shown in Fig. 3. A transition is apparent near 200 K with no divergence between ZFC and FC data, suggesting that the spin order is antiferromagnetic. The inverse susceptibility shows linear variation with temperature at 240–300 K, and a Curie–Weiss fit in this range gives a paramagnetic moment of 3.0 μB and a Weiss temperature of θ = −460 K. These values are in keeping with antiferromagnetically coupled Cr3+S = 3/2 spins (ideal paramagnetic moment 3.9 μB), given the limited fitting range for the parameters.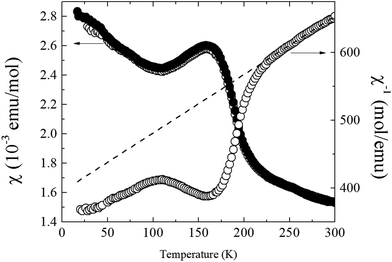 | ||
| Fig. 3 ZFC and FC magnetic susceptibilities for Ca2Cr2O5, and the inverse ZFC susceptibility showing a Curie–Weiss fit to the 240 < T < 300 K data. | ||
Magnetic diffraction peaks were observed in neutron profiles collected at 10 to 200 K. These peaks are indexed by magnetic propagation vector k = (111) and the possible irreducible representations (irreps) and basis vectors for spin order with this vector applied to the I2mb space group are shown in Table 3.
| Irreps | Basis vectors | m x , my, mz | |||
|---|---|---|---|---|---|
| (0, 0, 0) | (0, 1/2, 0) | (x, 1/4, z) | (x, 3/4, −z) | ||
| Γ1 | Ψ1 | 1, 0, 0 | −1, 0, 0 | 0, 1, 0 | 0, −1, 0 |
| Γ2 | Ψ2 | 1, 0, 0 | 1, 0, 0 | 1, 0, 0 | 1, 0, 0 |
| Ψ3 | 0, 0, 1 | 0, 0, −1 | |||
| Γ3 | Ψ4 | 0, 1, 0 | 0, −1, 0 | 1, 0, 0 | −1, 0, 0 |
| Ψ5 | 0, 0, 1 | 0, 0, 1 | 0, 0, 1 | 0, 0, 1 | |
| Γ4 | Ψ6 | 0, 1, 0 | 0, 1, 0 | 0, 1, 0 | 0, 1, 0 |
| Ψ7 | 0, 0, 1 | 0, 0, −1 | |||
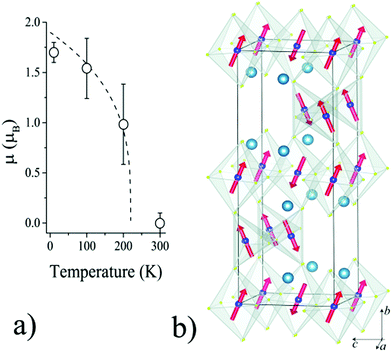
Fits using a single basis vector for the spin order did not account well for the magnetic intensities. However, a good fit (as shown for the 10 K data in Fig. 2) is obtained using a combination of the basis vectors Ψ5(Γ3) and Ψ6(Γ4). These describe a CyGz antiferromagnetic order of spins, following standard convention for magnetic order in perovskites.20 Although the Cr1 and Cr2 site moments are not symmetry-related, refining their spin components independently did not give a significantly improved fit over a model where their components were constrained to be the same, so the latter description was used as the final model. The my and mz moment components at 10 K have values of 1.08(5) and 1.33(7) μB, and the resultant moment is m = 1.7(1) μB. Neutron diffraction shows that the Néel temperature for Ca2Cr2O5 is >200 K (see inset to Fig. 2), and hence somewhat higher than the susceptibility features observed in Fig. 3. An estimate of TN ≈ 220 K is obtained by fitting the temperature variation of the moment in Fig. 4a with a critical law m(T) = m(0)[1 − (T/TN)]β for an exponent β ≈ 0.3, in keeping with theoretical models such as the three-dimensional XY magnet for which β = 0.34.
The magnetic structure of Ca2Cr2O5 consists of antiferromagnetic planes of Cr1 and Cr2 moments as shown in Fig. 4b. Spins lie in the bc-plane and are tilted by 51(4)° from the b-axis. Neighbouring Cr1 and Cr2 spins connected through Cr1–O–Cr2 bridges are canted by ∼100° suggesting that antisymmetric Dzialoshinskii–Moriya exchange is significant, given the absence of a centre of symmetry or other symmetry relations between Cr1 and Cr2, and that Cr1–O–Cr2 Heisenberg exchange interactions are weak or frustrated. This reduction in the low temperature ordered moment of 1.7 μB from the ideal value of 3 μB for 3d3 Cr3+ is also consistent with some frustration in the spin order. Such spin canting is unusual in brownmillerites, as most have simple collinear antiferromagnetic structures with moments parallel to a or b.
Conclusions
This study confirms that Ca2Cr2O5 synthesised by reduction of the high pressure perovskite phase CaCrO3 is a brownmillerite and adopts the ordered I2mb superstructure at ambient temperature. The unusual tetrahedral coordination of Cr3+ results in local structural distortions. The observation of Cr3+ in this unconventional environment illustrates the use of hard–soft chemistry to stabilise unusual coordination geometries. This brownmillerite material containing a high concentration of oxygen vacancies in cubic-perovskite (100) planes may offer high mobility for oxide ion transport, as was found in the hard–soft product SrCrO2.8.4,6 These stoichiometric reduced phases may also be useful model compounds to help understand oxide ion migration in chromium perovskite mixed conductors used in fuel cell anodes such as (La1−xSrx)(Cr1−yMy)O3−δ (M = Mn, Fe, Co, Ni).21Ca2Cr2O5 displays an antiferromagnetic spin ordering transition near 220 K, which is comparable to the 280 K Néel temperature for the perovskite LaCrO3. Substantial canting of moments in successive layers is observed, suggesting that symmetric Heisenberg exchange interactions are weak or frustrated so that competing antisymmetric Dzialoshinskii–Moriya interactions determine spin directions.
Acknowledgements
We thank Dr I. da Silva (ISIS) and Dr Elise Pachoud (Edinburgh) for assistance with diffraction measurements. We also thank EPSRC, STFC and the Royal Society for support.Notes and references
- M. A. Hayward, Semicond. Sci. Technol., 2014, 29, 064010 CrossRef
.
- A. J. Williams, A. Gillies, J. P. Attfield, G. Heymann, H. Huppertz, M. J. Martínez-Lopez and J. A. Alonso, Phys. Rev. B: Condens. Matter, 2006, 73, 104409 CrossRef
.
- L. Ortega-San-Martin, A. J. Williams, J. Rodgers, J. P. Attfield, G. Heymann and J. Huppertz, Phys. Rev. Lett., 2007, 99, 255701 CrossRef
.
- A. M. Arevalo-Lopez, J. A. Rodgers, M. S. Senn, F. Sher, J. Farnham, W. Gibbs and J. P. Attfield, Angew. Chem., Int. Ed., 2012, 51, 10791 CrossRef CAS PubMed
.
- A. M. Arevalo-Lopez, F. Sher, J. Farnham, A. J. Watson and J. P. Attfield, Chem. Mater., 2013, 25, 2346 CrossRef CAS
.
- K. H. L. Zhang, P. V. Sushko, R. Colby, Y. Du, M. E. Bowden and S. A. Chambers, Nat. Commun., 2014, 5, 4669 CAS
.
- A. M. Arevalo-Lopez, B. Liang, M. S. Senn, C. Murray, C. Tang and J. P. Attfield, J. Mater. Chem. C, 2014, 2, 9364 RSC
.
- M. A. Alario-Franco, E. Castillo-Martinez and A. M. Arevalo-Lopez, High Pressure Res., 2009, 29, 254 CrossRef CAS
.
- A. C. Komarek, S. V. Streltsov, M. Isobe, T. Moller, M. Hoelzel, A. Senyshyn, D. Trots, M. T. Fernandez-Diaz, T. Hansen, H. Gotou, T. Yagi, Y. Ueda, V. I. Anisimov, M. Gruninger, D. I. Khomskii and M. Braden, Phys. Rev. Lett., 2008, 101, 167204 CrossRef CAS
.
- Y. Bando, Y. Sekikawa, H. Yamamura and Y. Matsui, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1981, 37, 723 CrossRef
.
- E. F. Bertaut, P. Blum and A. Sagnieres, Acta Crystallogr., 1959, 12, 149 CrossRef
.
- I. Kontoulis and B. C. H. Steele, J. Eur. Ceram. Soc., 1992, 9, 459 CrossRef CAS
.
- T. G. Parsons, H. D'Hondt, J. Hadermann and M. A. Hayward, Chem. Mater., 2009, 21, 5527 CrossRef CAS
.
- C. B. Vanpeteghem, R. J. Angel, J. Zhao;, N. L. Ross, G. J. Redhammer and F. Seifert, Phys. Chem. Miner., 2008, 35, 493 CrossRef CAS PubMed
.
- V. Kahlenberg, R. X. Fischer and C. S. J. Shaw, Am. Mineral., 2000, 85, 1061 CAS
.
- V. Kahlenberg and C. S. J. Shaw, Z. Kristallogr., 2001, 216, 206 CrossRef CAS
.
- J. Zhang, H. Zheng, C. D. Malliakas, J. M. Allred, Y. Ren, Q. Li, T. H. Han and J. F. Mitchell, Chem. Mater., 2014, 26, 7172 CrossRef CAS
.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef
.
- T. G. Parsons, H. D'Hondt, J. Hadermann and M. A. Hayward, Chem. Mater., 2009, 21, 5527 CrossRef CAS
.
- E. O. Wollan and W. C. Koehler, Phys. Rev., 1955, 100, 545 CrossRef CAS
.
- P. I. Cowin, C. T. G. Petit, R. Lan, J. T. S. Irvine and S. Tao, Adv. Energy Mater., 2011, 1, 314 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |