Nanoscale metallogel via self-assembly of self-assembled trinuclear coordination rings: multi-stimuli-responsive soft materials†
Abstract
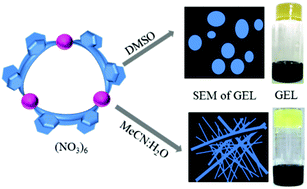
A rare variety of coordination rings having the M3L6 composition are prepared by the combination of Pd(NO3)2 with an imidazolyl or benzimidazolyl appended bidentate non-chelating ligand (i.e.L1 or L2). The variable concentration 1H NMR spectra of the trinuclear complexes [Pd3(L1)6](NO3)6, 1a and [Pd3(L2)6](NO3)6, 2a in DMSO-d6 provided valuable information on the self-assembly phenomenon of the already self-assembled complexes. Interestingly, the signals of 2a are broadened upon increasing the concentration. The solution of 2a in DMSO formed a supramolecular metallogel above a certain concentration (i.e. 2% w/v), however, complex 1a could not form any gel. This phenomenon is attributed to the presence of an auxiliary π-surface in the benzimidazole moiety in L2, in contrast to the imidazole moiety of L1. The influence of anions in gel formation was studied by preparing several samples using a variety of palladium(II) salts and L2 in DMSO. It was found that oxoanions like nitrate, perchlorate, triflate and tosylate are amicable for gel formation, probably due to their capabilities of forming H-bonds. The counter anions like tetrafluoroborate, hexafluorophosphate, or hexafluoroantimonate could not assist in the formation of gel. The stimuli-responsive nature of the gel and the reversible gel–sol conversion were demonstrated by dis-assembly and re-assembly processes of 2a as controlled by a pair of stimuli such as halide-nitrate, DMAP-HNO3, and ethylenediamine-Pd(NO3)2. No gel could be prepared by the combination of Ni(NO3)2 or Pt(NO3)2 with ligand L2. Thus, a subtle change in the ligand design, metal ion and counter anion is demonstrated as the responsible parameter for the construction of the three component multi-stimuli-responsive supramolecular gel.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...