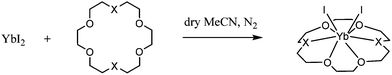Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
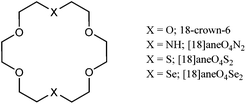
Divalent ytterbium complexes with crown and heterocrown ethers†‡
Philip N.
Bartlett
,
Martin J. D.
Champion
,
Mark. E.
Light
,
William
Levason
*,
Gillian
Reid
and
Peter W.
Richardson
School of Chemistry, University of Southampton, Southampton SO17 1BJ, UK. E-mail: wxl@soton.ac.uk
First published on 2nd December 2014
Abstract
Several unusual complexes of ytterbium(II) stabilised by coordination to mixed O4X2-donor macrocyclic ligands (X = O, NH, S or Se) are described. Distorted 8-coordination is evident from the crystal structure of the neutral [YbI2([18]aneO4Se2)], forming the first reported example of Yb(II)-selenoether coordination.
The chemistry of the lanthanides (4f elements) is dominated by the 3+ oxidation state,1 and apart from the fluorides and oxides of Pr4+ and Tb4+, only cerium forms an extensive range of compounds in the 4+ state.1–3 For many years lower oxidation states were limited to some divalent samarium, europium and ytterbium complexes, and compounds such as LaI2 or GdS, which were formulated as “metallic” Ln(III) species with the extra electron delocalised in a conduction band.2 Early uses of Eu(II) as a one electron reductant were followed by the development of a similar role for SmI2 and [(Cp*)2Sm(thf)2].4 Recent work aimed at developing suites of lanthanide(II) complexes with varying reactivities for use in organic reductions, couplings, polymerisation and dinitrogen activation has led to new organometallic complexes of all the M(II) (except for Pm) as [K(macrocycle)][Ln(C5H4SiMe3)3] (macrocycle = 18-crown-6 or 222-crypt),§ although samarium(II) reagents remain the most widely used in organic chemistry.5
Reaction of anhydrous YbI2 with the ligands 18-crown-6, [18]aneO4S2, [18]aneO4Se2 and [18]aneO4N2 in anhydrous MeCN gave modest yields of white to pale yellow species, [YbI2(ligand)] (Scheme 1); the formulation was confirmed by microanalysis.
The IR spectra showed the presence of the ligand and absence of MeCN (and H2O). The complexes are moderately soluble in anhydrous MeCN, and very readily hydrolysed and oxidised by exposure to air. Very small crystals of [YbI2([18]aneO4Se2)] were obtained after storing an MeCN solution of the complex in a freezer (−18 °C).
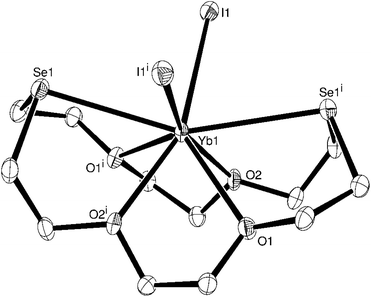
The complex has crystallographic two-fold symmetry and is isomorphous with [CaI2([18]aneO4Se2)],8 based upon hexadentate coordination of the macrocycle, with two mutually cis iodide ligands completing the distorted 8-coordination at Yb(II) (Fig. 1). The bond lengths and angles are also very similar to the Ca(II) species. This is as expected given their similar ionic radii (Yb2+ = 1.16 Å, Ca2+ = 1.14 Å).9 The neutral [YbI2([18]aneO4Se2)] molecule is also structurally very similar to the cation in the isoelectronic [LuI2([18]aneO4Se2)]I·2MeCN,6 albeit with slightly shorter corresponding bonds (by ∼0.1–0.15 Å) in the latter, reflecting the smaller radius resulting from the higher charge (Lu3+ ionic radius 1.00 Å). In the Yb(II) complex, the Se–M–Se angle is some 13° wider than in the Lu(III) analogue, and the I–M–I angle ∼5° wider. The larger La3+ ion (ionic radius = 1.22 Å)9 forms nine-coordinate [LaI3(macrocycle)] with these hexadentate macrocycles.6
Although we were unable to obtain diffraction-quality crystals of [YbI2(18-crown-6)], [YbI2([18]aneO4S2)] or [YbI2([18]aneO4N2)], on the basis of microanalytical and spectroscopic data it seems highly probable that they also contain eight-coordinate Yb(II).
The complexes are very poorly soluble in CH2Cl2, however, they are moderately soluble in MeCN, affording yellow solutions. 1H NMR spectra of [YbI2([18]aneO4S2)] and [YbI2([18]aneO4Se2)] in anhydrous CD3CN show broad OCH2 and S(Se)CH2 resonances, with smaller coordination shifts than observed in the corresponding Lu(III) cations.6 We were unable to observe a 77Se NMR resonance from [YbI2([18]aneO4Se2)], contrasting with [LuI2([18]aneO4Se2)]I which exhibited a sharp resonance with a low frequency coordination shift (Δ) of −32 ppm. The NMR data are consistent with the macrocycles exhibiting dynamic processes in solution and weaker binding to the Yb(II), compared with the isoelectronic Lu(III) analogues. In 10−3 mol dm−3 solutions in anhydrous MeCN, the complexes have high molar conductivities, in the range characteristic of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytes,10 which suggests that like the Ln(III) analogues,6 the iodide ligands are displaced by MeCN in dilute solution, although MeCN is not present in the solid complexes isolated from this solvent.
2 electrolytes,10 which suggests that like the Ln(III) analogues,6 the iodide ligands are displaced by MeCN in dilute solution, although MeCN is not present in the solid complexes isolated from this solvent.
Cyclic voltammetry experiments were performed to probe the redox responses from these complexes. Owing to their poor solubility in non-coordinating solvents such as CH2Cl2, electrochemistry experiments were initially attempted using 0.1 mol dm−3 [NnBu4]I in anhydrous MeCN as supporting electrolyte. However, the presence of excess iodide significantly suppressed the solubility of the complexes. Therefore, subsequent experiments used 0.1 mol dm−3 [NnBu4][BF4]–MeCN as supporting electrolyte. Under these conditions the complexes each show voltammetric peaks attributed to the electro-oxidation of free iodide. The relative peak heights indicated the liberation of one iodide ion per Yb(II) complex. No other peaks associated with Yb(II)/(III) redox couples were evident under these conditions over the potential range −2 to +1.5 V.
Experimental
Details of the spectroscopic and electrochemical measurements and X-ray crystallography are given in the ESI.‡Synthesis
Conclusions
The synthesis, spectroscopic and structural characterisation of rare heterocrown complexes of a divalent lanthanide, specifically Yb(II), has been achieved. Coordination of soft thioether and selenoether functions to Yb(II) has been demonstrated. The properties of these complexes are intermediate between those of Ln(III) and Ca(II)/Sr(II) analogues. More generally, although the coordination of the soft donors is clearly aided by the presence of O-donors in the macrocycle, the study suggests that a wider range of coordination complexes derived from divalent lanthanides with neutral ligands, including other soft Lewis bases, should be accessible with judicious choice of solvent, reaction conditions and anion.Acknowledgements
We thank EPSRC for support (EP/1033394/1). The SCFED Project (http://www.scfed.net) is a multidisciplinary collaboration of British universities investigating the fundamental and applied aspects of supercritical fluids.Notes and references
- S. Cotton, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2004, vol. 4, p. 93 Search PubMed.
- D. A. Johnson, Adv. Inorg. Chem. Radiochem., 1977, 20, 1 CrossRef CAS.
- N. A. Piro, J. R. Robinson, P. J. Walsh and E. J. Schelter, Coord. Chem. Rev., 2014, 260, 21 CrossRef CAS.
- (a) W. J. Evans, Coord. Chem. Rev., 2000, 206–207, 263 CrossRef CAS; (b) M. N. Bochkarev, Coord. Chem. Rev., 2004, 248, 835 CrossRef CAS.
- (a) M. Szostak and D. J. Procter, Angew. Chem., Int. Ed., 2012, 51, 9238 CrossRef CAS PubMed; (b) M. R. MacDonald, J. E. Bates, J. W. Ziller, P. Furche and W. J. Evans, J. Am. Chem. Soc., 2013, 135, 9857 CrossRef CAS PubMed.
- M. J. D. Champion, P. Farina, W. Levason and G. Reid, Dalton Trans., 2013, 42, 13179 RSC.
- (a) W. Levason, M. L. Matthews, B. Patel, G. Reid and M. Webster, Dalton Trans., 2004, 3305 RSC; (b) S. D. Reid, A. L. Hector, W. Levason, G. Reid, B. J. Waller and M. Webster, Dalton Trans., 2007, 4769 RSC.
- P. Farina, W. Levason and G. Reid, Dalton Trans., 2013, 42, 89 RSC.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, Wiley, New York, 6th edn, 1999 Search PubMed.
- 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Electrolytes have ΛM (MeCN 10−3 mol dm−3) ∼120–160 and 1
1 Electrolytes have ΛM (MeCN 10−3 mol dm−3) ∼120–160 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytes ∼220–300 Ω−1 cm2 mol−1. W. J. Geary, Coord. Chem. Rev., 1971, 6, 81 CrossRef.
2 electrolytes ∼220–300 Ω−1 cm2 mol−1. W. J. Geary, Coord. Chem. Rev., 1971, 6, 81 CrossRef.
Footnotes |
| † Dedicated to the memory of Professor Ken Wade FRS in recognition of his many contributions to inorganic chemistry. |
| ‡ Electronic supplementary information (ESI) available: Further experimental and crystallographic details for the structure of [YbI2([18]aneO4Se2)]. CCDC 1024072. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt03462g |
| § [18]aneO4S2 = 1,4,10,13-tetraoxa-7,16-dithiacyclooctadecane, [18]aneO4Se2 = 1,4,10,13-tetraoxa-7,16-diselenacyclooctadecane, [18]aneN2O4 = 1,4,10,13-tetraoxa-7,16-diazacyclooctadecane, 18-crown-6 = 1,4,7,10,13,16-hexaoxacyclooctadecane. |
| This journal is © The Royal Society of Chemistry 2015 |