Open Access Article
Open Access ArticleFacile synthesis of [(NHC)MX(cod)] and [(NHC)MCl(CO)2] (M = Rh, Ir; X = Cl, I) complexes†
R.
Savka
and
H.
Plenio
*
Organometallic Chemistry, Alarich-Weiss-Str. 12, Fachbereich Chemie, TU Darmstadt, 64287 Darmstadt, Germany. E-mail: plenio@tu-darmstadt.de
First published on 12th November 2014
Abstract
The reactions of [MCl(cod)]2 (M = Rh, Ir) with different NHC·HX (X = Cl, I), K2CO3 in technical grade acetone under air provide simple access to various [(NHC)MX(cod)] complexes; a facile one-pot synthesis of [(NHC)MCl(CO)2] (M = Rh, Ir) is also reported.
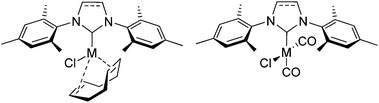
The use of N-heterocyclic carbenes has a profound impact on organometallic chemistry and homogeneous catalysis and during the last few years metal complexes across the periodic table have been synthesized.1 [(NHC)RhCl(cod)] (cod = 1,5-cyclooctadiene) and [(NHC)RhCl(CO)2] complexes were first reported by the Lappert group,2 later Herrmann studied such complexes with Rh and Ir in some detail.3 Catalytic applications in transfer hydrogenation,4 hydrogenation,5 hydrosilylation6 or hydrogenation reactions for magnetization transfer7 are known. More important is the use of [(NHC)MCl(cod)] and [(NHC)MCl(CO)2] (M = Rh, Ir) (Scheme 1) for the determination of the electron donating properties (Tolman electronic parameter) of the respective NHC ligands,8 since Nolan et al.9 and Crabtree et al.10 have established the use of such complexes as practical alternatives to the traditional LNi(CO)3 complexes.11 Consequently, for a large proportion of newly prepared NHC ligands the respective [(NHC)MCl(cod)] and [(NHC)MCl(CO)2] (M = Rh, Ir) have been synthesized.12
[(NHC)MX(cod)] (M = Rh, Ir) complexes (Scheme 1) are prepared along two different routes: (a) the direct reaction of the isolated or the in situ generated carbene (obtained from the treatment of the azolium salt with a strong base) with [MCl(cod)]2 appears to be the most popular approach.12 Sometimes there is a different order in the addition of the reactants, such that [MCl(cod)]2 is first treated with an alkoxide base (e.g. KOtBu, NaOMe) to generate the basic [M(OR)(cod)]2 complex, followed by addition of the azolium salt.13 Whatever approach is chosen, anaerobic conditions are mandatory, furthermore base sensitive functional groups may cause problems. (b) Alternatively, transmetalation reactions of [MCl(cod)]2 with preformed Ag-NHC complexes10,14 or other NHC transfer reagents like imidazolium carboxylates,15 2-(pentafluorophenyl)imidazolidines16 or imidazolidines17 have been employed. However, the generation of the respective Ag(NHC) salt or of the other NHC transfer reagents requires additional synthetic steps. The synthesis of the respective [(NHC)MCl(CO)2] complexes relies on [(NHC)MCl(cod)], whose solutions are treated with CO gas, again this constitutes an additional reaction step.12f
Simplicity in the synthesis of such complexes is essential for the application of the respective NHC-metal complexes. Therefore, we want to present here the facile one-step synthesis of [(NHC)MX(cod)] (X = Cl, I) and [(NHC)MCl(CO)2] (M = Rh, Ir) under ambient atmosphere utilizing [MCl(cod)]2 as the metal source. Our approach is motivated by recent publications from Gimeno et al.18 and Nolan et al.,19 who independently reported simple reaction conditions (acetone or CH2Cl2 and K2CO3 as the base) for the synthesis of various NHC-Au complexes from [AuCl(Me2S)] or NHC-Cu complexes from CuX (X = Cl, Br, I).20
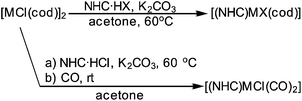
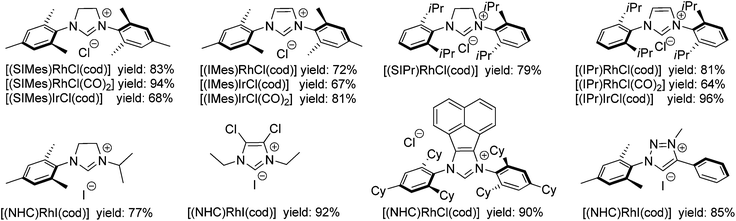
The reaction of [RhCl(cod)]2 with SIMes·HCl using K2CO3 as the base in acetone was tested first and found to provide the desired [(SIMes)RhCl(cod)] in an isolated yield of 83% (Scheme 2). In order to establish the generality of this approach seven additional azolium salts were tested (Scheme 3). The nature of the NHC precursors was modulated (saturated vs. unsaturated; N,N′-diaryl vs. N,N′-aryl,alkyl vs. N,N′-dialkyl, backbone substituted and abnormal carbene) to cover a larger range of different NHC ligands. The respective eight different [(NHC)RhX(cod)] complexes were obtained in 72–92% yield. Notably, for all of the tested reactions, there is a larger than 90% conversion into [(NHC)RhX(cod)]. However, due to the small scale nature of the reactions (typically ca. 50–100 mg of the product were formed), the isolated yields can be slightly lower, due to losses during work-up. The NHC transfer to Rh works equally well for the azolium salt with an iodide counter ion and leads to the isolation of the respective [(NHC)RhI(cod)] complexes.
Next the reactions of [IrCl(cod)]2 and azolium salts leading to [(NHC)IrCl(cod)] were studied, by applying the same conditions as for the Rh complexes. For the unsaturated azolium salts (IMes·HCl, IPr·HCl) good yields of the respective [(NHC)IrCl(cod)] were obtained, employing the “Rh conditions”. However, for the related saturated azolium salts (SIMes·HCl) additional optimization of the reaction conditions was required. The amount of base was found to be a critical parameter and the near stoichiometric use of the base (1.2 equiv.) gives better results (Scheme 3) than the 3 equiv. K2CO3 used for the synthesis of the Rh complexes.
Based on the high yielding conversion of [MCl(cod)]2 (M = Rh, Ir) into the respective [(NHC)MX(cod)], we became also interested in whether in situ generated [(NHC)MCl(cod)] complexes can be reacted directly with CO to render the respective [(NHC)MCl(CO)2] complexes (Scheme 2). This one-pot approach turned out to be successful and several [(NHC)MCl(CO)2] (Scheme 3) were isolated in 64–94% yield. Due to the lower solubility of the respective carbonyl complexes, the isolated yields of those complexes can be higher than those of the related cod complexes.
In order to get some insight into the mechanism of the facile [(NHC)MX(cod)] formation, SIMes·HCl was treated with K2CO3 in acetone. In the absence of metal complexes, this reaction slowly led to the respective ring-opened formamides, which are known decomposition products of the free NHC ligands in the presence of moisture.21 Nonetheless, we consider it unlikely that the [(NHC)MX(cod)] complexes are formed via free carbenes, since free carbenes are unstable in acetone. Therefore the reaction of [RhCl(cod)]2 with K2CO3 in acetone was studied, which leads to the formation of a yellow precipitate. This solid was isolated and reacted with SIMes·HCl. Notably, in the absence of additional base, the formation of [(SIMes)RhCl(cod)] was observed. This precipitate appears to be the key species in the synthesis and it has to contain a basic group. Based on the literature data the yellow solid can either be the carbonato complex22 or the hydroxo complex [Rh(OH)(cod)]2.23 The latter complex was reported to form in 95% yield from [RhCl(cod)2] in water/KOH.23 Neither the IR spectrum of the yellow material nor its 13C NMR provide evidence for the formation of the carbonato complex. Instead, the 1H NMR and the IR spectrum display characteristic signals for the well known [Rh(OH)(cod)]2.23 Based on this we assume that the primary product of the reaction is the hydroxo complex, which directly reacts with an azolium salt to form the respective NHC-M (M = Rh, Ir) complexes. The better stability of such complexes with Rh than with Ir23 may explain the slightly better synthetic results obtained for the Rh complexes.
In conclusion, we have demonstrated the facile one-step synthesis of [(NHC)MX(cod)] (M = Rh, Ir; X = Cl, I) and the simple one-pot synthesis of [(NHC)MCl(CO)2] complexes for different azolium salts. The desired complexes are produced in excellent yields with different NHC ligands. The reaction of the metal precursor [MCl(cod)]2 with the respective azolium salt is carried out in technical grade acetone under ambient atmosphere and provides easy access to the respective NHC complexes – for the synthesis of the respective carbonyl complexes, CO is bubbled through the crude reaction mixture.
General procedure for the synthesis of [(NHC)RhX(cod)] (X = Cl, I), and [(NHC)IrCl(cod)] complexes: a vial was charged, under air, with the corresponding NHC·HX (1 equiv.), [MCl(cod)]2 (0.5 equiv.) and K2CO3 (3 equiv.). The resulting mixture was suspended in acetone (3 mL) and stirred for 20 h at 60 °C (for one-pot synthesis of carbonyl complexes, continue below). After this time the volatiles were removed in vacuo and CH2Cl2 was added (3 mL). The mixture was filtered through a pad of silica. The pad of silica was washed with CH2Cl2 until the filtrate becomes colorless and the product dried in vacuo.
One-pot synthesis of [(NHC)MCl(CO)2] (M = Rh, Ir): next, the mixture was cooled to room temperature and carbon monoxide bubbled through under vigorous stirring for 20 min. The volatiles were removed in vacuo, the residue dissolved in CH2Cl2 and filtered through Celite, which was washed with CH2Cl2 until the filtrate becomes colorless. The volatiles were removed in vacuo and the residue washed with pentane (5 mL). The pale yellow precipitate was collected by filtration, washed with pentane and dried in vacuo.
The authors gratefully acknowledge support of this work by the DFG via DFG 178/13-2. We wish to thank one referee for useful literature references.
Notes and references
- N-Heterocyclic Carbenes, ed. S. P. Nolan, Wiley-VCH, Weinheim, 2014 Search PubMed.
- (a) M. J. Doyle and M. F. Lappert, J. Chem. Soc., Chem. Commun., 1974, 679–680 RSC; (b) J. E. Hill and T. A. Nile, J. Organomet. Chem., 1977, 137, 293–300 CrossRef CAS; (c) M. J. Doyle, M. F. Lappert, P. L. Pye and P. Terreros, J. Chem. Soc., Dalton Trans., 1984, 2355–2364 RSC.
- (a) W. A. Herrmann, M. Elison, J. Fischer, C. Köcher and G. R. J. Artus, Chem. – Eur. J., 1996, 2, 772–780 CrossRef CAS; (b) W. A. Herrmann, J. Schütz, G. D. Frey and E. Herdtweck, Organometallics, 2006, 25, 2437–2448 CrossRef CAS.
- S. Gülcemal, A. G. Gökçe and B. Çetinkaya, Inorg. Chem., 2013, 52, 10601–10609 CrossRef PubMed.
- E. L. Kolychev, S. Kronig, K. Brandhorst, M. Freytag, P. G. Jones and M. Tamm, J. Am. Chem. Soc., 2013, 135, 12448–12459 CrossRef CAS PubMed.
- A. Monney and M. Albrecht, Chem. Commun., 2012, 48, 10960–10962 RSC.
- M. J. Cowley, R. W. Adams, K. D. Atkinson, M. C. R. Cockett, S. B. Duckett, G. G. R. Green, J. A. B. Lohman, R. Kerssebaum, D. Kilgour and R. E. Mewis, J. Am. Chem. Soc., 2011, 133, 6134–6137 CrossRef CAS PubMed.
- (a) S. Wolf and H. Plenio, J. Organomet. Chem., 2009, 694, 1487–1492 CrossRef CAS PubMed; (b) T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef PubMed.
- D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC.
- A. R. Chianese, X. Li, M. C. Janzen, J. W. Faller and R. H. Crabtree, Organometallics, 2003, 22, 1663–1667 CrossRef CAS.
- (a) R. Dorta, E. D. Stevens, N. M. Scott, C. Costabile, L. Cavallo, C. D. Hoff and S. P. Nolan, J. Am. Chem. Soc., 2005, 127, 2485–2495 CrossRef CAS PubMed; (b) C. A. Tolman, Chem. Rev., 1977, 77, 313–348 CrossRef CAS.
- (a) C. D. Varnado, E. L. Rosen, M. S. Collins, V. M. Lynch and C. W. Bielawski, Dalton Trans., 2013, 42, 13251–13264 RSC; (b) A. Makhloufi, W. Frank and C. Ganter, Organometallics, 2012, 31, 2001–2008 CrossRef CAS; (c) H. Valdés, M. Poyatos and E. Peris, Organometallics, 2013, 32, 6445–6451 CrossRef; (d) V. César, N. Lugan and G. Lavigne, Eur. J. Inorg. Chem., 2010, 2010, 361–365 CrossRef; (e) S. Dierick, D. F. Dewez and I. E. Markó, Organometallics, 2014, 33, 677–683 CrossRef CAS; (f) S. Leuthäußer, D. Schwarz and H. Plenio, Chem. – Eur. J., 2007, 13, 7195–7203 CrossRef PubMed; (g) Y. Borguet, G. Zaragoza, A. Demonceau and L. Delaude, Dalton Trans., 2013, 42, 7287–7296 RSC; (h) A. Fürstner, M. Alcarazo, H. Krause and C. W. Lehmann, J. Am. Chem. Soc., 2007, 129, 12676–12677 CrossRef PubMed; (i) M. Mayr, K. Wurst, K.-H. Ongania and M. R. Buchmeiser, Chem. – Eur. J., 2004, 10, 1256–1266 CrossRef CAS PubMed; (j) M. Iglesias, D. J. Beetstra, A. Stasch, P. N. Horton, M. B. Hursthouse, S. J. Coles, K. J. Cavell, A. Dervisi and I. A. Fallis, Organometallics, 2007, 26, 4800–4809 CrossRef CAS; (k) R. D. Savka and H. Plenio, J. Organomet. Chem., 2012, 710, 68 CrossRef CAS PubMed; with anhydrous K2CO3; (l) T. Sato, Y. Hirose, D. Yoshioka and S. Oi, Organometallics, 2012, 31, 6995–7003 CrossRef CAS.
- (a) M. T. Zamora, M. J. Ferguson, R. McDonald and M. Cowie, Organometallics, 2012, 31, 5463–5477 CrossRef CAS; (b) O. Torres, M. Martín and E. Sola, Organometallics, 2009, 28, 863–870 CrossRef CAS; (c) I. Özdemir, B. Yigit, B. Çetinkaya, D. Ülkü, M. N. Tahir and C. Arıcı, J. Organomet. Chem., 2001, 633, 27–32 CrossRef.
- (a) A. R. Chianese, A. Kovacevic, B. M. Zeglis, J. W. Faller and R. H. Crabtree, Organometallics, 2004, 23, 2461–2468 CrossRef CAS; (b) S. Saravanakumar, A. I. Oprea, M. Kindermann, P. G. Jones and J. Heinicke, Chem. – Eur. J., 2006, 12, 3143–3154 CrossRef CAS PubMed.
- A. M. Voutchkova, M. Feliz, E. Clot, O. Eisenstein and R. H. Crabtree, J. Am. Chem. Soc., 2007, 129, 12834–12846 CrossRef CAS PubMed.
- A. P. Blum, T. Ritter and R. H. Grubbs, Organometallics, 2007, 26, 2122–2124 CrossRef CAS.
- A. Prades, M. Poyatos, J. A. Mata and E. Peris, Angew. Chem., Int. Ed., 2011, 50, 7666–7669 CrossRef CAS PubMed.
- R. Visbal, A. Laguna and M. C. Gimeno, Chem. Commun., 2013, 49, 5642–5644 RSC.
- A. Collado, A. Gomez-Suarez, A. R. Martin, A. M. Slawin and S. P. Nolan, Chem. Commun., 2013, 49, 5541–5543 RSC.
- O. Santoro, A. Collado, A. M. Z. Slawin, S. P. Nolan and C. S. J. Cazin, Chem. Commun., 2013, 49, 10483–10485 RSC.
- M. Emin Günay, N. Özdemir, M. Ulusoy, M. Uçak, M. Dinçer and B. Çetinkaya, J. Organomet. Chem., 2009, 694, 2179–2184 CrossRef PubMed.
- E. G. Lundquist, K. Folting, J. C. Huffman and K. G. Caulton, Inorg. Chem., 1987, 26, 205–208 CrossRef CAS.
- R. Uson, L. A. Oro, J. A. Cabeza, H. E. Bryndza and M. P. Stepro, Inorg. Synth., 1985, 23, 126–130 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and full characterisation of complexes. See DOI: 10.1039/c4dt03449j |
| This journal is © The Royal Society of Chemistry 2015 |