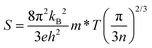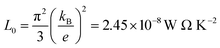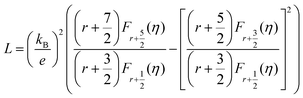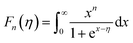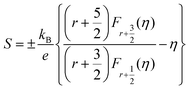Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermoelectric properties and chlorine doping effect of In4Pb0.01Sn0.03Se2.9Clx polycrystalline compounds
Jin
Hee Kim
a,
Min
Jae Kim
a,
Suekyung
Oh
a,
Jong-Soo
Rhyee
*a,
Su-Dong
Park
b and
Docheon
Ahn
c
aDepartment of Applied Physics and Institute of Natural Sciences, Kyung Hee University, Yongin 446-701, Korea. E-mail: jsrhyee@khu.ac.kr; Fax: +82-31-204-8122; Tel: +82-31-201-2415
bAdvanced Electrical Materials Group, Korea Electrotechnology Research Institute, Changwon 641-600, Korea
cPohang Accelerator Laboratory (PAL), Pohang 790-784, Korea
First published on 22nd December 2014
Abstract
We investigated the thermoelectric properties of Cl-doped polycrystalline compounds In4Pb0.01Sn0.03Se2.9Clx (x = 0.02, 0.04, and 0.06). X-ray diffraction measurement shows a gradual change in lattice volume for x ≤ 0.04 without any impurity phases indicating a systemic change in Cl doping. The Cl doping in the compounds has the effect of increasing carrier concentration and the effective mass of carriers, resulting in an increase in power factor at a high temperature (∼700 K). Because of the increased electrical conductivity at a high temperature, the dimensionless thermoelectric figure of merit ZT reaches 1.25 at 723 K for the x = 0.04 Cl-doped compound, which is a relatively high value for n-type polycrystalline materials.
1. Introduction
The global energy crisis makes a case for not only the development of new eco-friendly energy sources but also efficient consumption of the energy currently in use. Thermoelectric research is one of the efforts made to promote energy efficiency. Thermoelectric performance can be defined by the dimensionless figure of merit ZT = S2σT/κ, where S, σ, κ, and T are the Seebeck coefficient, electrical conductivity, thermal conductivity, and absolute temperature, respectively.State-of-the-art thermoelectric materials have reported high ZT values of 2.6 at 923 K (SnSe)1 and 2.2 at 915 K (SrTe-doped PbTe).2 However, the materials with high recorded ZT values are mostly based on p-type materials. We need counterpart n-type materials with high thermoelectric performance in order to make thermoelectric devices.
One class of promising n-type thermoelectric materials is In4Se3-based compounds. Single-crystalline In4Se3−x (x = 0.65) reported a high ZT value of 1.48 at 705 K using the Peierls distortion.3
Basically, because the Peierls distortion is based on quasi-one-dimensional electron transport, the effect of the Peierls distortion is averaged out in polycrystalline materials. Therefore, compared with single-crystalline materials, polycrystalline In4Se3−x compounds show decreased ZT values of 0.63 at 710 K and 0.97 at 698 K, synthesized by spark plasma sintering (SPS) and ball milling-hot press sintering methods, respectively.4,5
Recently, Pb and Sn co-doping of In4Se3-based compounds produced greatly increased values of thermoelectric performances in spite of polycrystalline compounds. For example, Pb/Sn-co-doped In4PbxSnySe3 and Se-deficient In4Pb0.01Sn0.03Se3−x compounds exhibit relatively high ZT values of 1.4 at 733 K and 1.2 (x = 0.1) at 723 K, respectively.6,7
The other promising route to improve thermoelectric properties in In4Se3 compounds is halogen doping of the compounds In4Se3−xHy (H = F, Cl, Br, and I).8 The Hall mobilities of single-crystalline In4Se3−xH0.03 are significantly increased by halogen doping. It was found that Cl doping is the most effective for increasing Hall mobility. Cl-doped single-crystalline In4Se3−xCl0.03 (x = 0.33) exhibits high ZT values, with a maximum ZT of 1.53 at 698 K, over a wide temperature range compared with In4Se3−x.9 The critical issue with In4Se3-based compounds is the increase in chemical potential because the materials have far from the optimum chemical potential from a Boltzmann transport calculation.3,9 It has been reported that multiple cation doping and halogen doping on a Se-deficient site can increase the chemical potential resulting in enhancement of thermoelectric performance.6,10,11
Here, we investigate the Cl doping effect on Pb/Sn-co-doped In4Pb0.01Sn0.03Se2.9Clx compounds. In the previous study, we successfully synthesized polycrystalline In4Pb0.01Sn0.03Se2.9 compounds without any impurities which exhibited a maximum ZT value of 1.2 at 723 K.7 However, the power factor of the In4Pb0.01Sn0.03Se2.9 compound can increase further, because it is still far from the optimized chemical potential of 0.8 eV from a Boltzmann transport calculation.3,9 The chemical potential of the In4Se3 phase can be further increased by electron doping. Cl doping is believed to be a good candidate for increasing thermoelectric performance in multiple elements-doped In4Pb0.01Sn0.03Se2.9Clx compounds.
2. Experimental
Polycrystalline ingots of In4Pb0.01Sn0.03Se2.9Clx (x = 0.02, 0.04, and 0.06) were synthesized by melting, quenching, and annealing processes. The elements In, Pb, Sn, and Se, and InCl3 compound were sealed in evacuated quartz tubes under high vacuum with respect to their stoichiometric molar ratios. The quartz ampoules were heated at 1123 K for 24 h with subsequent water quenching.The ingots were pulverized and sintered under an argon atmosphere by hot press at about 730 K for 1 h under uniaxial pressure of 70 MPa. In order to increase their thermal stability, the pellets were annealed at 753 K for 5 days. The relative densities of the annealed samples were above 95% (5.82–5.93 g cm−3) of the theoretical density.
The crystal structure and phase of the annealed samples were identified by powder X-ray diffraction (XRD) using Cu Kα radiation (D8 Advance, Bruker). Rietveld refinement was carried out using FullProf software. The electrical resistivity ρ and Seebeck coefficient S were measured under an argon atmosphere by a four-probe method using a thermoelectric properties measurement system (ZEM-3, ULVAC-RIKO, Japan). The carrier concentration was calculated by the relation nH = −1/(RHe) where RH is the Hall coefficient. The Hall coefficient RH (RH = ρxy/H) and Hall resistivity ρxy were measured by a four-probe contact method with sweeping magnetic fields from −5 T to +5 T using a physical property measurement system (PPMS, Quantum Design, USA). We obtained the thermal conductivity from the relation κ = λρsCp, where λ, ρs, and Cp are the thermal diffusivity, sample density, and specific heat, respectively. The thermal diffusivity λ and specific heat Cp were measured by a laser flash method (LFA-457, NETZSCH) and physical property measurement system, respectively.
3. Results and discussion
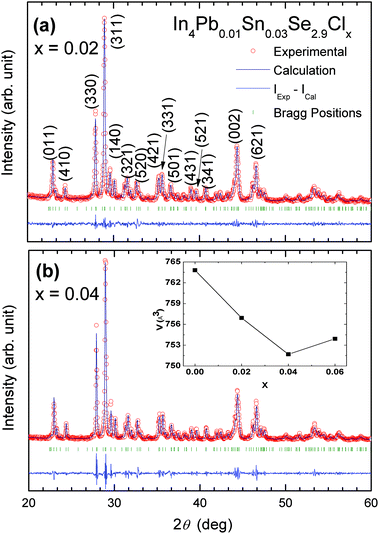
Fig. 1 shows Rietveld refinement analysis on powder X-ray diffraction (XRD) patterns of the prepared samples (x = 0.02 and x = 0.04) based on the orthorhombic Pnnm space group, respectively. The XRD patterns show a single phase of In4Se3 with no noticeable impurity peaks except for x = 0.06. Because of the impurity phase of InSe (hexagonal, no. 194), we cannot analyse the XRD pattern by Rietveld refinement for the x = 0.06 compound. This indicates that the x = 0.06 compound is at the solubility limit of chlorine which induces the phase separation from In4Se3 to the InSe phase. The lattice parameter was calculated as shown in Table 1. In the previous investigation of Se-deficient Pb/Sn-co-doped compounds In4Pb0.01Sn0.03Se3−x (x = 0.1, 0.2, 0.3, 0.4, and 0.5), the lattice parameters were systematically changed.7 However, the lattice parameters of the Cl-doped samples In4Pb0.01Sn0.03Se2.9Clx do not exhibit systematic changes but show a decrease in lattice volume (inset of Fig. 1b) with increasing chlorine concentration up to the x = 0.04 compound. Because the compound with x = 0.06 has an InSe impurity phase, the lattice volume of this compound does not exhibit systematic change.From the formation energy calculations of halogen-substituted In4Se3−xH0.03 compounds, substitution and vacancy occupation at a Se3 site by Cl doping is the most stable state in In4Se3−x.8 The decrease in lattice volume by Cl doping can be understood by the strong Coulomb interaction from the electronegativity of Cl and the Cl substitution at a Se3 site.12
The temperature-dependent electrical resistivity of polycrystalline In4Pb0.01Sn0.03Se2.9Clx (x = 0.02, 0.04, and 0.06) compounds and the x = 0.0 compound7 is presented in Fig. 2(a). The electrical resistivity of the Cl-doped compounds exhibits semiconducting behaviour in the measured temperature range. The increasing electrical resistivity with increasing Cl doping shows similar behaviour to In4Se2.7Clx.10
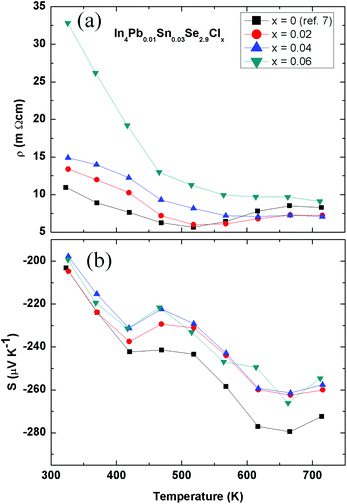 | ||
| Fig. 2 Temperature-dependent electrical resistivity ρ(T) (a) and Seebeck coefficient S(T) (b) of In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04, and 0.06) compounds. | ||
The electrical resistivity is given by the relation ρ = neμ, where n, e, and μ are the Hall carrier concentration, electric charge, and Hall mobility of the carrier. From Hall resistivity ρxy and electrical resistivity ρ measurements, we obtain the effective carrier concentration nH and Hall mobility μH, which are listed in Table 2. The carrier concentration of the In4Pb0.01Sn0.03Se2.9Clx (x = 0.02) compound shows a value 1.58 times higher than that of the pristine In4Pb0.01Sn0.03Se2.9 compound. The carrier concentration does not increase with Cl doping for x = 0.04 and 0.06, implying a solubility limit of the compounds as in the case of polycrystalline In4Se2.7Clx compounds (x = 0–0.05).10
| x | n H (×1018 cm−3) | ρ (mΩ cm) | μ H (cm2 V−1 s−1) | −S (μV K−1) | m* (me) |
|---|---|---|---|---|---|
| 0.00 | 2.67 | 10.9 | 214 | 203 | 0.18 |
| 0.02 | 4.22 | 13.4 | 111 | 205 | 0.25 |
| 0.04 | 4.09 | 14.9 | 103 | 198 | 0.24 |
| 0.06 | 3.99 | 32.9 | 48 | 199 | 0.23 |
The Hall mobilities of In4Pb0.01Sn0.03Se2.9Clx compounds decrease with increasing Cl doping. In contrast with the result for an In4Se3−xCl0.03 single crystal,9 the polycrystalline compounds In4Se2.7Clx10 and In4Pb0.01Sn0.03Se2.9Clx exhibited a decreased Hall mobility by increasing the Cl doping. This implies that the Hall mobility of polycrystalline In4Se3 is decreased by excess Cl impurity scattering at the grain boundary. However, a CuBr-doped In4Se2.5 polycrystal shows an increased Hall mobility.13
The increase in Hall mobility in bulk crystalline materials is caused by an enhancement of crystallinity, e.g. in a chlorine-doped In4Se3−xCl0.03 single crystal.9 For example, X-ray diffraction patterns for the cross-sectional planes of crystalline ingots show well-aligned crystal orientation. However, for polycrystalline materials, the Hall mobility of chlorine-doped samples decreases with increasing chlorine doping concentration.10 This implies that the chlorine itself acts as a scattering centre of carriers but helps to enhance crystallinity during crystal growth. Doped chlorine can increase the Hall carrier density, but excess chlorine can precipitate at grain boundaries, resulting in a decrease in Hall mobility. On the other hand, single-crystalline materials have less effect on grain boundaries, and thereby the enhanced crystallinity can increase the Hall mobility of carriers.
The temperature-dependent Seebeck coefficient S(T) of polycrystalline In4Pb0.01Sn0.03Se2.9Clx (x = 0.02, 0.04, and 0.06) compounds decreases with Cl doping as compared with the x = 0.0 compound, as shown in Fig. 2(b). The Seebeck coefficient of degenerate semiconductors can be expressed by the following equation:
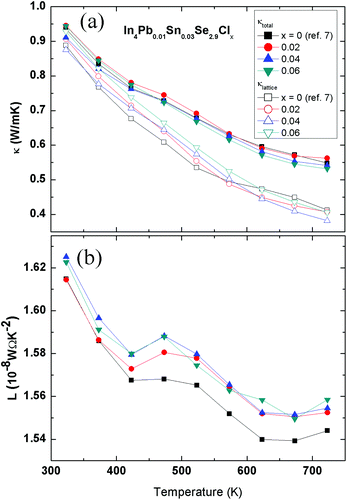
The total thermal conductivities of the polycrystalline In4Pb0.01Sn0.03Se2.9Clx (x = 0, 0.02, 0.04 and 0.06) compounds are shown in Fig. 3(a). The thermal conductivities of the x = 0.0, 0.02, 0.04, and 0.06 compounds decrease with increasing temperature. This conventional 1/T behaviour is mainly caused by an acoustic phonon contribution to thermal transport. There is no systematic change in κ(T) with respect to the Cl doping concentration.
In general, the total thermal conductivity κ is composed of electronic κel and lattice thermal conductivity κph. The electronic thermal conductivity κel can be calculated by the Wiedemann–Franz law κel = L0σT, where L0, σ, and T are the Lorenz number, electrical conductivity, and absolute temperature, respectively. In usual cases, the Lorenz number is written as:
However, the Lorenz number is incorrect in correlated metals and many degenerate semiconductors. In order to obtain a more reliable Lorenz number, we calculated the Lorenz number using the following equation:7
In most cases, the scattering parameter for acoustic phonon scattering is r = −1/2. When we fit the measured Seebeck coefficient to the following equation with a free parameter η, we can obtain the Fermi integral η:
Using the Fermi integral, the calculated temperature-dependent Lorenz numbers of In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04, and 0.06) are shown in Fig. 3(b). The calculated temperature-dependent Lorenz number is lower than the conventional Lorenz number L0 = 2.45 × 10−8 W Ω K−2. The low Lorenz numbers indicate that the electronic contribution to thermal transport is lower than in conventional metals. Using the calculated Lorenz number and electrical resistivity, we can obtain the lattice thermal conductivity by subtracting the electronic thermal conductivity from the total thermal conductivity as shown in Fig. 3(a) (open symbols). The change in lattice thermal conductivity with respect to the chlorine doping concentration of In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04, and 0.06) is not obvious. The similar thermal conductivity can be explained by the similarity of the crystal structure and phonon dispersion relation between In4Se3 and InSe.16
Fig. 4(a) shows the temperature-dependent power factor S2σ of polycrystalline In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04, and 0.06) compounds. The decreased power factor with increasing Cl doping is mainly caused by decreased Hall mobility in the room- and mid-temperature range. On the other hand, slightly increased power factors for the x = 0.02 and 0.04 compounds are obtained near 723 K. The x = 0.06 compound exhibits a decreased power factor from that of the other samples (x = 0.0, 0.02, and 0.04) implying that InSe phase separation is ineffective in increasing the power factor in the In4Se3 phase.
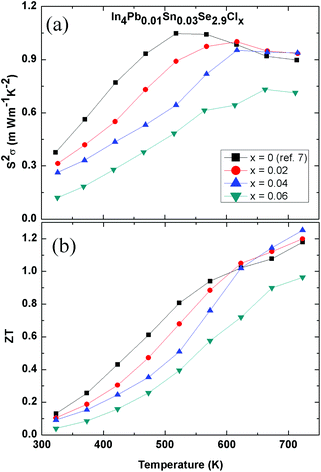 | ||
| Fig. 4 Temperature-dependent power factor S2σ (a) and ZT values (b) of In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04 and 0.06) compounds. | ||
The ZT values of polycrystalline In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04, and 0.06) compounds are presented in Fig. 4(b). The maximum ZT, when x = 0.04, reaches 1.25 at 723 K, which is a slightly increased value compared with that of the pristine compound x = 0.0 (ZT = 1.2). The high ZT value over a wide temperature range in bulk crystals of In4Se3−xCl0.03 (1.53) is caused by a significant increase in Hall mobility which is attributed to an enhancement of crystallinity by chlorine doping.9 Because the polycrystalline In4Se2.7Clx samples exhibit a decreased ZT value (0.67) with increasing chlorine doping concentration, we believe that a further enhancement of ZT is possible in In4Pb0.01Sn0.03Se2.9Clx compounds in single-crystalline form. Therefore we should investigate the thermoelectric properties of the single-crystalline compounds as further research.
4. Conclusions
Cl-doped polycrystalline In4Pb0.01Sn0.03Se2.9Clx (x = 0.0, 0.02, 0.04, and 0.06) compounds are synthesized well by melting and solid-state reactions with a systematic change in lattice volume. Because of chlorine impurity scattering, the electrical resistivities of the Cl-doped samples (x = 0.02, 0.04, and 0.06) are increased as compared with the non-Cl-doped sample (x = 0.0) near room temperature, which is mainly caused by a decrease in Hall mobility by chlorine doping. However, the electrical resistivities of the x = 0.02 and 0.04 compounds are decreased at high temperature accompanied by smearing out of the broad shoulder near 670 K.Consequently, they exhibit a relatively low thermal conductivity and high power factor over a wide temperature range. The power factor and ZT values of the Cl-doped compounds (x = 0.02 and 0.04) increased as compared with the non-Cl-doped compound (x = 0.0) near 700 K. The maximum ZT value of 1.25 is relatively high for polycrystalline In4Se3-based compounds. Further enhancement of thermoelectric performance can be expected in single-crystalline compounds by promoting crystallinity of the samples.
Acknowledgements
This research was supported by Mid-career Research Program (Strategy) (no. 2012R1A2A1A03005174) and Nano-Material Technology Development Program (2011-0030147) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology and Energy Efficiency & Resources program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Knowledge Economy (no. 20112010100100), and SK Innovation.Notes and references
- L.-D. Zhao, S.-H. Lo, Y. Zhang, H. Sun, G. Tan, C. Uher, C. Wolverton, V. P. Dravid and M. G. Kanatzidis, Nature, 2014, 508, 373 CrossRef CAS PubMed.
- K. Biswas, J. He, I. D. Blum, C.-I. Wu, T. P. Hogan, D. N. Seidman, V. P. Dravid and M. G. Kanatzidis, Nature, 2012, 489, 414 CrossRef CAS PubMed.
- J.-S. Rhyee, K. H. Lee, S. M. Lee, E. Cho, S. I. Kim, E. Lee, Y. S. Kwon, J. H. Shim and G. Kotliar, Nature, 2009, 459, 965 CrossRef CAS PubMed.
- J.-S. Rhyee, E. Cho, K. H. Lee, S. M. Lee, S. I. Kim, H.-S. Kim, Y. S. Kwon and S. J. Kim, Appl. Phys. Lett., 2009, 95, 212106 CrossRef PubMed.
- G. H. Zhu, Y. C. Lan, H. Wang, G. Joshi, Q. Chen and Z. F. Ren, Phys. Rev. B: Condens. Matter, 2011, 83, 115201 CrossRef.
- Z.-S. Lin, L. Chen, L.-M. Wang, J.-T. Zhao and L.-M. Wu, Adv. Mater., 2013, 25, 4800 CrossRef CAS PubMed.
- J. H. Kim, M. J. Kim, S. Oh and J.-S. Rhyee, J. Alloys Compd., 2014, 615, 933 CrossRef CAS PubMed.
- K. Ahn, E. Cho, J.-S. Rhyee, S. I. Kim, S. Hwang, H.-S. Kim, S. M. Lee and K. H. Lee, J. Mater. Chem., 2012, 22, 5730 RSC.
- J.-S. Rhyee, K. Ahn, K. H. Lee, H. S. Ji and J.-H. Shim, Adv. Mater., 2011, 23, 2191 CrossRef CAS PubMed.
- J.-S. Rhyee and D. Choi, J. Appl. Phys., 2011, 110, 083706 CrossRef PubMed.
- K. Ahn, E. Cho, J.-S. Rhyee, S. I. Kim, S. M. Lee and K. H. Lee, Appl. Phys. Lett., 2011, 99, 102110 CrossRef PubMed.
- R. T. Sanderson, J. Am. Chem. Soc., 1983, 105, 2259 CrossRef CAS.
- Y. Luo, J. Yang, G. Liu, Y. Xiao, L. Fu, W. Li, P. Zhu, J. Peng, S. Gao and J. Zhang, Adv. Energy Mater., 2014, 4, 1300599 Search PubMed.
- A. F. Ioffe, Semiconductor Thermoelements and Thermoelectric Cooling, Infosearch, 1957 Search PubMed.
- H. J. Goldsmid and J. W. Sharp, J. Electron. Mater., 1999, 28, 869 CrossRef CAS.
- M. Sznajder, K. Z. Rushchanskii, L. Y. Kharkhalis and D. M. Bercha, Phys. Status Solidi B, 2006, 243, 592 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |