Direct synthesis of size-tunable PbS nanocubes and octahedra and the pH effect on crystal shape control†
Abstract
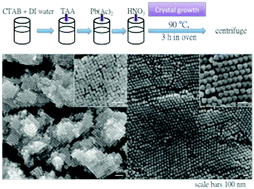
A one-pot synthesis for the growth of PbS nanocubes and octahedra has been developed by heating an aqueous mixture of cetyltrimethylammonium bromide (CTAB), thioacetamide (TAA), lead acetate, and nitric acid at 90 °C for 3 h. The method allows for direct large-scale production of small and uniform PbS nanocubes. The PbS cubes and octahedra exhibit different surface properties, as evidenced by zeta potential measurements and stability tests in methyl orange and methylene blue solutions. Examination of the reagent introduction sequence indicates that early or late addition of nitric acid to tune the initial solution pH can strongly influence crystal growth rate and result in the formation of PbS crystals with different sizes and shapes. Remarkably, the use of pre-formed PbS nanocubes for further crystal growth under low TAA concentration can transform the cubes into large octahedra through a series of particle shape evolution.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...