A new insight into the chemistry of iridium(iii) complexes bearing phenyl phenylphosphonite cyclometalate and chelating pyridyl triazolate: the excited-state proton transfer tautomerism via an inter-ligand PO–H⋯N hydrogen bond†
Abstract
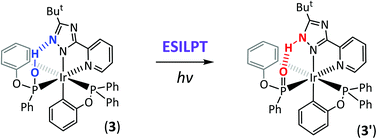
Treatment of [IrCl3(tht)3], where tht = tetrahydrothiophene, with two equiv. of phenyl diphenylphosphinite (pdpitH) gave [Ir(pdpitH)(pdpit)(tht)Cl2] (1), which on further reaction with 3-t-butyl-5-(2-pyridyl)-1,2,4-triazole (bptzH) and NaOAc using a one-pot reaction afforded [Ir(pdpit)2(bptz)] (2). In sharp contrast, the reaction of [IrCl3(tht)3], pdpitH, and bptzH in the presence of a stronger base, Na2CO3, afforded a phenyl phenylphosphonite (pppo)-containing Ir(III) complex [Ir(pdpit)(pppo)(bptz)] (3) that reveals a strong PO–H–N inter-ligand hydrogen bond (H-bond), as evidenced by the single crystal X-ray structural analysis. For confirmation, addition of diazomethane to a diethylether solution of 3 led to the isolation of two methylated Ir(III) isomeric complexes, i.e. [Ir(pdpit)(pppoMe)(bptz)] (4) and [Ir(pdpit)(pppo)(bptzMe)] (5), possessing either a PO–Me or N–Me bonding fragment, respectively. The absorption spectrum of 3 in CH2Cl2 resembles that of 4, implying the dominant PO–H character in solution. Despite the prevailing PO–H character both in the solid crystal and in solution, its corresponding emission resembles that of 5, leading us to propose a mechanism incorporating the excited-state inter-ligand proton transfer (ESILPT) from PO–H to N–H isomeric form via the pre-existing PO⋯H⋯N hydrogen bond. The thermodynamics of proton transfer tautomerism are discussed on the basis of absorption/emission spectroscopy in combination with computational approaches; additional support is given by the relationship between emission pattern versus the position of protons and methyl substituents. The results demonstrate for the first time a paradigm of excited-state proton transfer for the transition metal complexes in the triplet manifold.
- This article is part of the themed collection: Luminescent Complexes and Materials for Light-Emitting Devices


 Please wait while we load your content...
Please wait while we load your content...