Actinide selectivity of 1,10-phenanthroline-2,9-dicarboxamide and its derivatives: a theoretical prediction followed by experimental validation†
Abstract
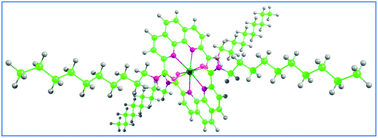
The conventional concept of selective complexation of actinides with soft donor ligands (either S or N donor) has been modified here through exploiting the concept, “intra-ligand synergism”, where a hard donor atom, such as oxygen preferentially binds to trivalent actinides [An(III)] as compared to the valence iso-electronic trivalent lanthanides [Ln(III)] in presence of another soft donor centre. We have theoretically predicted the selectivity of 1,10-phenanthroline-2,9-dicarboxylamide towards the Am(III) ion through density functional calculations. Subsequently, several such amide derivatives have been synthesized to optimize the solubility of the ligands in the organic phase. Finally, solvent extraction experiments have been carried out to validate our theoretical prediction on the selectivity of mixed donor ligands towards Am(III) as compared to Eu(III), and a maximum separation factor of about 51 has been achieved experimentally using the 2,9-bis(N-decylaminocarbonyl)-1,10-phenanthroline ligand.

 Please wait while we load your content...
Please wait while we load your content...