Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nucleophile-mediated oxa-Michael addition reactions of divinyl sulfone – a thiol-free option for step-growth polymerisations†
Simone
Strasser
and
Christian
Slugovc
*
Institute for Chemistry and Technology of Materials, Graz University of Technology, NAWI Graz, Stremayrgasse 9, A-8010 Graz, Austria. E-mail: slugovc@tugraz.at
First published on 8th October 2015
Abstract
Triphenylphosphine and 4-dimethylaminopyridine promote the oxa-Michael addition reaction of alcohols and divinyl sulfone. Under solvent-free conditions, the reaction is particularly fast and allows for the preparation of polymers.
The Michael addition between thiols and electron-poor olefins such as (meth)acrylates, maleimides, unsaturated ketones, or vinyl sulfones is categorized as a click reaction1 and finds wide application in polymer and materials chemistry in both versions, the base-catalysed and the nucleophile-mediated pathways.2 Of particular interest is the nucleophile-mediated thiol-Michael addition which proceeds via a pathway different from base-catalysed reactions:3 the nucleophile adds to an electron-deficient vinyl group, generating a strongly basic carbon-centred anion deprotonating the thiol, which then initiates the thiol-Michael addition catalytic cycle. In this context, we became interested whether it is feasible to use less acidic4 and less nucleophilic alcohols instead of thiols. The substitution of thiols with alcohols would be desirable for the fact that much more alcohols are readily (and commercially) available than thiols. Moreover, inherent drawbacks of thiols, such as their tendency to lead to oxidative disulfide formation, their (often) bad odour and toxicity as well as their propensity to oxidize to give sulfones and sulfoxides in the final materials could be circumvented by using alcohols instead of thiols. The development of oxa-Michael reactions in general has received less attention compared to the addition of nucleophiles based on carbon, nitrogen or sulphur to electron-poor olefins, but received considerable attention in the past decade.5 In particular, the work by Bergman and Toste on phosphine-initiated hydroalkoxylation of α,β-unsaturated ketones paved the way for studying and using the oxa-Michael reaction in organic chemistry.6 The application of these findings in polymer and materials chemistry, however, is scarce.7 We herein wish to report the nucleophile-mediated oxa-Michael addition reaction of divinyl sulfone and alcohols and the application of this reaction in polymerisations.
First, a series of potential nucleophiles for mediating the reaction was tested, employing the reaction conditions outlined in Scheme 1 and Table 1. 1-Methylimidazole (1-MIM) and 1,4-diazabicyclo[2.2.2]octane (DABCO) as well as triethylamine (NEt3) gave hardly any conversion towards the desired oxa-Michael addition product (cf.Table 1, entries 1–3). 4-Dimethylaminopyridine (DMAP) performed better yielding roughly a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of mono- and disubstituted products after 24 h (cf.Table 1, entry 4). Finally, triphenylphosphine (PPh3) gave satisfactory results.9 After 2 h, 83% of the disubstituted product were already observed and full conversion towards this product was found after 24 h (cf.Table 1, entry 5). The PPh3 loading could be reduced to 2.5 mol% without compromising the conversion too much (91% disubstituted product after 24 h). For comparison, a base-mediated reaction was carried out using 3 eq. (with respect to DVS) of Cs2CO3. Under these conditions, after 2 h, less than 2/3 of DVS were converted into the disubstituted addition product and after 24 h, almost complete conversion was observed (cf.Table 1, entry 6). The reactivity order of the different nucleophiles could be rationalized with their corresponding methyl cation affinities (MCA, cf.Table 1).8 Based on these results, we selected PPh3 as the nucleophilic mediator for further studies because PPh3 is air-stable (in contrast to electron-richer alkylphosphines which exhibit higher MCA values8 and accordingly putatively a higher reactivity).
1 mixture of mono- and disubstituted products after 24 h (cf.Table 1, entry 4). Finally, triphenylphosphine (PPh3) gave satisfactory results.9 After 2 h, 83% of the disubstituted product were already observed and full conversion towards this product was found after 24 h (cf.Table 1, entry 5). The PPh3 loading could be reduced to 2.5 mol% without compromising the conversion too much (91% disubstituted product after 24 h). For comparison, a base-mediated reaction was carried out using 3 eq. (with respect to DVS) of Cs2CO3. Under these conditions, after 2 h, less than 2/3 of DVS were converted into the disubstituted addition product and after 24 h, almost complete conversion was observed (cf.Table 1, entry 6). The reactivity order of the different nucleophiles could be rationalized with their corresponding methyl cation affinities (MCA, cf.Table 1).8 Based on these results, we selected PPh3 as the nucleophilic mediator for further studies because PPh3 is air-stable (in contrast to electron-richer alkylphosphines which exhibit higher MCA values8 and accordingly putatively a higher reactivity).
 | ||
| Scheme 1 Addition of benzyl alcohol (3 eq.) to divinyl sulfone (DVS) yielding the mono- (mono) and the diaddition (di) products initiated by various nucleophiles. | ||
| Mono/diadductb (%) | |||||
|---|---|---|---|---|---|
| Entry | Alcohol | Nuc | MCAa (kJ mol−1) | 2 h | 24 h |
| a Methyl cation affinity (MCA) according to ref. 8. b Conversion of DVS towards the mono- and disubstituted products as determined by 1H-NMR spectroscopy after reaction times of 2 h and 24 h. c 3 eq. Cs2CO3 with respect to DVS were used. | |||||
| 1 | R = CH2Ph | 1-MIM | 550 | 3/0 | 14/0 |
| 2 | R = CH2Ph | DABCO | 562 | <1/0 | 3/<1 |
| 3 | R = CH2Ph | NEt3 | 562 | 0/0 | 1/0 |
| 4 | R = CH2Ph | DMAP | 581 | 42/9 | 53/47 |
| 5 | R = CH2Ph | PPh3 | 618 | 17/83 | <1/>99 |
| 6 | R = CH2Ph | Cs2CO3c | — | 38/57 | 1/99 |
As the next step, the substrate scope of the reaction was investigated. Primary aliphatic alcohols (Table 2, entries 1–4) reacted faster than secondary alcohols (Table 2, entries 5 and 6). The simplest tertiary aliphatic alcohol, t-butanol, gave no reaction at all (Table 2, entry 7). Phenylmethanol is a better substrate than 2-propen-1-ol (cf.Table 2, entries 9 and 11) and 2-propyn-1-ol is a particularly good substrate (Table 2, entry 13). Adding alkyl groups at the 3- or 1-position of 2-propyn-1-ol (Table 2, entries 14–16) resulted in lower conversion compared to that of the parent substrate, while the secondary alcohol derivative, 1-phenyl-2-propyn-1-ol, gave similar results (Table 2, entry 17). A second phenyl group at the 1-position, however, is detrimental to high conversions under the studied reaction conditions. Nevertheless, the reactions shown in entries 16 and 18 in Table 1 make it clear that also tertiary alcohols with lower pKa values than t-butanol undergo the oxa-Michael addition reaction. Finally, phenol was shown to be a poor substrate under these reaction conditions.6 It is worth noting that the diadduct originating from methanol has been tested as an electrolyte in Li-ion batteries and is characterized by a wide electrochemical stability window (more than 5.0 V vs. Li/Li+).10 The reaction presented herein constitutes a fast and simple way towards such similar sulfone derivatives.
| Mono/diadductc (%) | |||||
|---|---|---|---|---|---|
| Entry | Alcohol, R = | pKaa | pKab | 2 h | 24 h |
| a According to ref. 4. b Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02, retrieved from SciFinder. c Conversion of DVS towards the mono- and disubstituted product as determined by 1H-NMR spectroscopy after reaction times of 2 h and 24 h; isolated yields of the diadducts range from 91 to 32% and are given in the ESI. d Additional products formed to about 17%. | |||||
| 1 | Me | 15.20 | 15.17 | 1/99 | <1/>99 |
| 2 | Et | 15.50 | 15.24 | 23/77 | 4/96 |
| 3 | n-Bu | 15.92 | 15.24 | 54/46 | 22/78 |
| 4 | n-Dodecyl | 15.20 | 73/7 | 66/34 | |
| 5 | i-Pr | 15.70 | 15.31 | 64/10 | 76/13 |
| 6 | c-Hex | 16.57 | 15.31 | 9/0 | 36/0 |
| 7 | t-Bu | 16.84 | 15.38 | 0/0 | 0/0 |
| 8 | Water | 15.7 | 31/9 | 34/13d | |
| 9 | Benzyl | 15.44 | 14.36 | 17/83 | <1/>99 |
| 10 | α-Methyl benzyl | 14.43 | 77/4 | 86/10 | |
| 11 | Allyl | 15.52 | 14.43 | 19/81 | 11/89 |
| 12 | 1-Phenyl allyl | 13.61 | 73/27 | 46/54 | |
| 13 | Propargyl | 13.60 | 13.21 | <1/>99 | <1/>99 |
| 14 | 3-Methyl propargyl | 14.16 | 13.14 | 36/64 | 22/78 |
| 15 | 1-i-Pr propargyl | 13.14 | 48/52 | 23/77 | |
| 16 | 1,1-Dimethyl propargyl | 13.34 | 65/14 | 67/33 | |
| 17 | 1-Phenyl propargyl | 12.40 | <1/>99 | <1/>99 | |
| 18 | 1,1-Diphenyl propargyl | 11.58 | 84/16 | 63/37 | |
| 19 | Phenol | 9.97 | 9.86 | 4/0 | 21/2 |
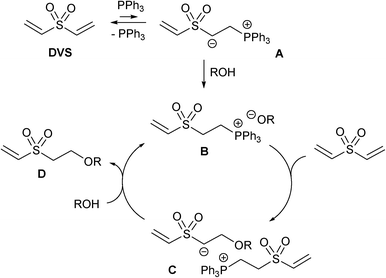
A mechanistic rationale of the reaction is shown in Scheme 2. Initial PPh3 conjugate addition11 to DVS results in the formation of zwitterion A which is detracted from the chemical equilibrium upon protonation by the alcohol, forming the corresponding phosphonium alkoxide B. The conjugate addition of the generated alkoxide to DVS forms ion pair C. Protonation of the carbanion by another alcohol results in the formation of the β-alkoxy sulfone derivative and phosphonium alkoxide B to complete the catalytic cycle. The rate-determining step of the reaction is believed to be the proton transfer from the alcohol to carbanion A (statement based on mechanistic studies of related thiol-Michael reactions).3,12 The values of entropy of activation (measured in a related system) are very negative suggesting the necessity of a precise arrangement of PPh3, the electron-deficient olefin and the proton donor for the reaction to occur.13 The following results support the briefly sketched mechanistic picture. Deuterium incorporation in α-position to the sulfone group was found upon performing the reaction with MeOH-d4 or in CDCl3 as the solvent (cf. ESI†), suggesting that a strong base is generated during the reaction. 31P-{1H}-NMR monitoring of the reaction revealed that a phosphorus signal for zwitterion A is not observable. Only upon addition of the alcohol signals at 24.5 ppm and 24.4 ppm (relative to 85% H3PO4) tentatively assigned to the phosphonium-containing ion pairs B and C formed.14 The reaction becomes faster by (a) using more acidic alcohols forming alkoxides with sufficient nucleophilicity (cf.Table 2), (b) lowering the reaction temperature (optimum about 10 °C, cf. ESI†) and (c) increasing the concentration (cf. ESI†). The latter finding implies to carry out the reaction under solvent-free conditions. The reaction of DVS and 2-propanol is a good showcase. Under the conditions, as mentioned in Table 2 (entry 5), only 13% of the diadduct were formed after 24 h. Optimized reaction conditions (using 26 eq. of 2-propanol and 10 mol% PPh3 at room temperature, cf. ESI†) gave the diadduct in 75% isolated yield after column chromatographic purification.
Switching to di- and trifunctional alcohols allowed for the preparation of polymers (cf.Fig. 1). Reacting an equimolar formulation of 4-(2-hydroxyethyl)phenol and DVS in water (5 eq.) upon adding 10 mol% PPh3 (stock solution in CH2Cl2) at 25 °C gave a polymer characterized by a number average molecular mass (Mn) of 780 g mol−1 and a polydispersity index (PDI) of 1.64. Similarly, using ethane-1,2-diol or but-2-yne-1,4-diol, polymers characterized by a Mn of 790 g mol−1 and a PDI of 1.5 or a Mn of 3200 g mol−1 and a PDI of 1.9 were obtained. In the case of but-2-yne-1,4-diol, a solution polymerisation reaction in THF/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ([DVS] = 0.5 mol L−1) was performed, yielding a polymer characterized by a Mn of 6400 g mol−1 and a PDI of 1.7 in 70% yield (conversion was quantitative, cf. ESI†).
1 ([DVS] = 0.5 mol L−1) was performed, yielding a polymer characterized by a Mn of 6400 g mol−1 and a PDI of 1.7 in 70% yield (conversion was quantitative, cf. ESI†).
Multifunctional alcohols like propane-1,2,3-triol and 2-ethyl-2-(hydroxymethyl)propane-1,3-diol gave insoluble yet cross-linked polymer networks. In these cases, solvent-free conditions were applied resulting in fast and exothermic reactions, thus mixing of the three components is hardly possible (also because of the poor solubility of PPh3 in the neat alcohols – ideally, the nucleophile should be dissolved in the alcohol and this solution should then be mixed with DVS15). Therefore, these reactions are preferably mediated with alcohol-soluble DMAP (0.05 eq.). Mixing of the DMAP/alcohol solution with DVS led to a somewhat retarded polymerisation reaction with a pot life of approx. 30 s. The formulation was transferred into Teflon moulds (22 × 5 × 3 mm) and specimens for dynamic mechanical analysis (DMA) were produced by curing for 4 h at 80 °C. The use of propane-1,2,3-triol resulted in stiff and brittle specimens which break upon mounting into the sample holder of the DMA machine. 2-Ethyl-2-(hydroxymethyl)propane-1,3-diol-based polymers gave specimens with a storage modulus of 3300 MPa at 10 °C and a Tg of 28 °C (determined to be the maximum of the loss modulus curve).
In conclusion, we demonstrated that the nucleophile-mediated oxa-Michael reaction between alcohols and divinyl sulfone is particularly fast and efficient under solvent-free conditions, allowing for the preparation of polymers. The reactivity of the alcohols decreases in the order primary > secondary > phenol > tertiary alcohols, and allylic, benzylic and propargylic alcohols exhibit distinctly higher reactivity than their saturated congeners.
Acknowledgements
The authors thank Isabel Hanghofer and Angelina Eder for the skillful synthetic work, Adriana Kovalcik for DMA analyses and Petra Kaschnitz for NMR analyses.Notes and references
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- (a) A. B. Lowe, Polym. Chem., 2014, 5, 4820–4870 RSC; (b) D. P. Nair, M. Podgorski, S. Chatani, T. Gong, W. Xi, C. R. Fenoli and C. N. Bowman, Chem. Mater., 2014, 26, 724–744 CrossRef CAS.
- (a) S. Chatani, D. P. Nair and C. N. Bowman, Polym. Chem., 2013, 4, 1048–1055 RSC; (b) J. W. Chan, C. E. Hoyle, A. B. Lowe and M. Bowman, Macromolecules, 2010, 43, 6381–6388 CrossRef CAS; (c) W. X. Xi, C. Wang, C. J. Kloxin and C. N. Bowman, ACS Macro Lett., 2012, 1, 811–814 CrossRef CAS.
- (a) I. Ugur, A. Marion, S. Parant, J. H. Jensen and G. Monard, J. Chem. Inf. Model., 2014, 54, 2200–2213 CrossRef CAS PubMed; (b) S. Takahashi, L. A. Cohen, H. K. Miller and E. G. Peake, J. Org. Chem., 1971, 36, 1205–1209 CrossRef CAS.
- C. F. Nising and S. Bräse, Chem. Soc. Rev., 2012, 41, 988–999 RSC.
- I. C. Steward, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2003, 125, 8696–8697 CrossRef PubMed.
- (a) Y. Yu and Y. Chau, Biomacromolecules, 2012, 13, 937–942 CrossRef CAS PubMed; (b) S.-I. Matsuoka, S. Namera and M. Suzuki, Polym. Chem., 2015, 6, 294–301 RSC.
- C. Lindner, R. Tandon, B. Maryasin, E. Larionov and H. Zipse, Beilstein J. Org. Chem., 2012, 8, 1406–1442 CrossRef CAS PubMed.
- H.-L. Liu, H. F. Jiang and Y.-G. Wang, Chin. J. Chem., 2007, 25, 1023–1026 CrossRef CAS PubMed.
- X.-G. Sun and C. A. Angell, Electrochem. Commun., 2005, 7, 261–266 CrossRef CAS PubMed.
- Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588–11619 RSC.
- C. Wang and C. Qi, Tetrahedron, 2013, 69, 5348–5354 CrossRef CAS PubMed.
- A. V. Salin, A. R. Faktkhutdinov, A. V. Il'in, V. I. Galkin and F. G. Shamsutdinova, Heteroat. Chem., 2014, 25, 205–216 CrossRef CAS PubMed.
- T. A. Albright, W. J. Freeman and E. E. Schweizer, J. Am. Chem. Soc., 1975, 97, 2942–2946 CrossRef CAS.
- S. Chatani, R. J. Sheridan, M. Podgorski, D. P. Nair and C. N. Bowman, Chem. Mater., 2013, 25, 3897–3901 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data. See DOI: 10.1039/c5cy01527h |
| This journal is © The Royal Society of Chemistry 2015 |