Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Subtle effects of ligand backbone on the efficiency of iron-diphos catalysed Negishi cross-coupling reactions†
Jamie
Clifton
a,
Evi R. M.
Habraken
b,
Paul G.
Pringle
*a and
Ian
Manners
a
aSchool of Chemistry, University of Bristol, Bristol BS8 1TS, UK. E-mail: paul.pringle@bristol.ac.uk
bDepartment of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam, The Netherlands
First published on 13th July 2015
Abstract
Substantially more active iron catalysts for a standard Negishi cross-coupling are obtained when bis(diarylphosphino)thiophenes are employed in place of the benchmark ligand bis(diphenylphosphino)benzene. The thiophene ligands have the advantages of ease of synthesis and ready modification.
There is great interest in the application of organoiron catalysts to reactions that have traditionally employed platinum group metal catalysts because of the potential advantages of lower expense and lower toxicity.1 Iron complexes have been found to be competent catalysts for the coupling of Grignard reagents with alkyl halides.2 Bedford et al.3 and Nakamura et al.4 have reported Fe-diphos catalysed Negishi couplings and recently Fe-monophos catalysed Negishi couplings.5 From the screening of several diphosphines, it has been established that Fe-catalysts for cross-coupling are sensitive to the ligand backbone and the P-substituents; in fact, the only diphos ligands that have produced significant activity and selectivity in Negishi couplings are derivatives of dpbz and, to a lesser degree, dppp and dppe (Chart 1).6 The mechanisms of Fe-catalysed cross-coupling reactions have been the subject of much discussion and an explanation of the ligand effects has yet to emerge.7
In view of the range of catalytic processes to which dpbz and its derivatives have been applied,8 it is surprising that diphosphinothiophenes have attracted little attention as ligands for catalysis,9 despite their ready synthesis and facility for modification compared to their dpbz analogues. Furthermore, thiophenes have garnered great interest because they are readily polymerised to give materials that have a raft of applications in the fields of organic LEDs and photovoltaics.10 Here the synthesis of a range of chelating diphosphinothiophenes is reported and it is shown that the Fe complexes of these ligands can match or outperform the best Fe-diphos catalysts in terms of activity and selectivity currently available for the coupling of benzyl halides with diarylzinc reagents.
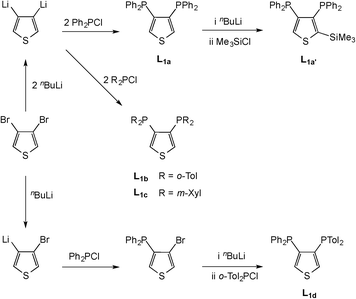
The routes to the nine new bis(diarylphosphino)thiophenes reported here are given in Schemes 1 and 2. The isomeric L1a and L2a were prepared as air-stable solids from the corresponding dibromothiophenes. The o-tolyl and m-xylyl derivatives L1b and L1c were made similarly from the corresponding chlorophosphines. The unsymmetrical ligand L1d was prepared in high purity by the sequence of lithiation-phosphination steps shown in Scheme 1. The introduction of Me3Si groups at the remaining unsubstituted thiophene sites in L1a and L2a to give L1a′ and L2a′ was readily achieved by the sequence of lithiation-silylation steps shown in Schemes 1 and 2. The ease of silylation of the backbone in the diphosphinothiophene contrasts with the inherent difficulty of functionalisation of diphosphinobenzenes.11
The ligands L1 and L2 have been tested in the Fe-catalysed Negishi coupling reaction shown in Scheme 3 and the results are given in Table 1 and shown graphically in Fig. 1. The three products are the desired cross-coupled product A and the two homocoupled species B and C. The yields of A and B have been calculated with respect to 3-methoxybenzyl halide since some of the zinc reagent is consumed in the formation of the catalyst; the byproduct C partly arises from the initial reduction of the Fe(II).
| Entry | Ligand | X | % conv. | A | B | C |
|---|---|---|---|---|---|---|
a Yields determined by 1H NMR spectroscopy with 1,3,5-trimethoxybenzene as internal standard. Catalyst pre-reduced3d and formed in situ. Reactions were run on a 0.5 mmol of substrate. L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio, 2 Fe ratio, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. X = Br: 45 °C, 4 h. X = Cl: 45 °C, 4 h. See ESI for details.
b Conditions as described in footnote a except the reaction was carried out at ambient temperatures.
c Conditions as described in footnote a but without addition of diphos ligand.
d Conditions as described in footnote a but without addition of FeCl2.
e Conditions as described in footnote a but with addition of [PdCl2(L1a)] in place of FeCl2/L1a. 1. X = Br: 45 °C, 4 h. X = Cl: 45 °C, 4 h. See ESI for details.
b Conditions as described in footnote a except the reaction was carried out at ambient temperatures.
c Conditions as described in footnote a but without addition of diphos ligand.
d Conditions as described in footnote a but without addition of FeCl2.
e Conditions as described in footnote a but with addition of [PdCl2(L1a)] in place of FeCl2/L1a.
|
||||||
| 1 | dpbz | Br | 100 | 98 | 2 | 10 |
| 2 | L1a | Br | 100 | 98 | 2 | 6 |
| 3 | L1b | Br | 69 | 56 | 13 | 20 |
| 4 | L1c | Br | 100 | 98 | 2 | 9 |
| 5 | L1d | Br | 98 | 83 | 15 | 19 |
| 6 | L1a′ | Br | 100 | 98 | 2 | 12 |
| 7 | L2a | Br | 100 | 99 | 1 | 10 |
| 8 | L2a′ | Br | 100 | 99 | 1 | 10 |
| 9 | dpbz | Cl | 76 | 76 | 0 | 8 |
| 10 | L1a | Cl | 98 | 98 | 0 | 6 |
| 11 | L1a′ | Cl | 92 | 92 | 0 | 19 |
| 12 | L1c | Cl | 100 | 100 | 0 | 6 |
| 13 | L2a | Cl | 92 | 92 | 0 | 5 |
| 14b | L1c | Cl | 100 | 100 | 0 | 10 |
| 15c | none | Cl | 37 | 35 | 2 | 70 |
| 16d | L1a | Cl | 0 | 0 | 0 | 2 |
| 17d | L1c | Cl | 0 | 0 | 0 | 2 |
| 18e | L1a | Cl | 6 | 0 | 6 | 15 |
 | ||
| Fig. 1 Plot of the conversions to products A and B from the Negishi cross-coupling shown in Scheme 3 as a function of ligand. | ||
The 3-methoxybenzyl bromide is the standard substrate3a for screening the Fe-diphos catalyzed Negishi coupling. From the results in Table 1, the following can be concluded. (1) The activity and selectivity of the catalysts derived from L1a (entry 2) and L2a (entry 7) are at least as good as those for the benchmark dpbz-Fe catalyst (entry 1). (2) Efficient catalysts are also generated with the ligands containing one SiMe3 group on the thiophene backbone, namely L1a′ (entry 5) and L2a′ (entry 8). (3) The catalysis is sensitive to the P-substituents: the o-tolyl groups in ligand L1b (entry 3) are deleterious to the catalysis whereas the xylyl groups in L1c (entry 4) are not. (4) The mixed PPh2/P(o-Tol)2 ligand L1d (entry 5) appears to produce a catalyst whose performance lies between that of the symmetrical congeners L1b/L1c.
Some of the best performing ligands from the above reaction screen have been compared against dpbz for the Negishi coupling with the more challenging 3-methoxybenzyl chloride (Scheme 3) and the results obtained are given in Table 1, entries 9–14. It is clear that all four new ligands significantly outperformed the dpbz in this catalysis with L1c (entry 12) giving a catalyst that produces quantitative conversion and >99.9% selectivity for the cross-coupled product. Remarkably, L1c gave quantitative conversion after 4 h when the catalysis was carried at ambient temperature (entry 14).
The validity of these results was tested with control experiments in which the reactions were carried out under the same conditions apart from: (i) no ligand was added (entry 15); (ii) no FeCl2 was added (entries 16 and 17); (iii) [PdCl2(L1a)] was added in place of FeCl2/L1a (entry 18). Only in the case of the FeCl2 with no diphos (entry 15) was some cross-coupling observed although selectivity was poor. The catalysis was also carried out with preformed [FeCl2(L1a)2] and the results obtained were the same as when the catalyst was formed in situ (entry 10).
In order to compare the relative rates of the dpbz-Fe catalyst with the highly efficient Fe/L1a Fe/L1c catalysts, the coupling of 3-methoxybenzyl chloride was monitored by 1H NMR spectroscopy as a function of time and the results are plotted in Fig. 2. It is evident from Fig. 2 that the reaction was 50% complete at ca. 75 min for the dpbz system, at ca. 13 min for L1a, and at <2 min for the L1c system. Estimates of the initial relative rates of conversion are thus 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 for dpbz
20 for dpbz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L1a
L1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L1c.
L1c.
 | ||
| Fig. 2 Conversion of 3-methoxybenzyl chloride with time for ligands L1a, L1c and dpbz. Aliquots of the reaction mixtures were quenched and conversions were determined from integration of the 1H NMR signals for the benzylic CH2 resonances for product A and 3-methoxybenzyl chloride. See the ESI† for full details. | ||
Conclusions
A new class of ligands for Fe-catalysed Negishi cross-coupling has been prepared featuring a thiophene backbone. The reactivity of the thiophene nucleus confers the advantages of ease and modularity of the synthesis of diphosphinothiophenes over their diphosphinobenzene analogues. In addition, it has been shown that the diphosphinothiophenes can produce Fe-catalysts that show significantly higher activity and selectivity than the dpbz analogues. Computational work is in progress that may shed light on the source of these ligand effects. In addition, thiophenes can be chemically or electrochemically polymerised to give conducting polymers and therefore the possibility of polymerising or co-polymerising the diphosphinothiophenes reported here is currently under investigation.Acknowledgements
We thank the Bristol Chemical Synthesis Centre for Doctoral Training, funded by EPSRC (EP/G036764/1), and the University of Bristol for a PhD studentship (to JC). We thank Professor Robin Bedford for useful discussions, Prof. E. J. M. Hensen (Eindhoven University of Technology) for support and the Regional Leonardo Bureau for an Erasmus grant (to ERMH).Notes and references
- For leading references see: (a) M. Tamura and J. K. Kochi, J. Am. Chem. Soc., 1971, 93, 1487 CrossRef CAS; (b) G. A. Molander, B. J. Rahn, D. C. Shubert and S. E. Bonde, Tetrahedron Lett., 1983, 5449 CrossRef CAS; (c) G. Cahiez and H. Avedissian, Synthesis, 1998, 1199 CrossRef CAS; (d) C. Bolm, J. Legros, J. LePaih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed; (e) S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3317 CrossRef CAS PubMed; (f) I. Sapountzis, W. Lin, C. C. Kofink, C. Despotopoulou and P. Knochel, Angew. Chem., Int. Ed., 2005, 44, 1654 CrossRef CAS PubMed; (g) P.-H. Phua, L. Lefort, J. A. F. Boogers, M. Tristany and J. G. de Vries, Chem. Commun., 2009, 3747 RSC; (h) K. Junge, K. Schröder and M. Beller, Chem. Commun., 2011, 47, 4849 RSC; (i) E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS PubMed; (j) K. Gopalaiah, Chem. Rev., 2013, 113, 3248 CrossRef CAS PubMed; (k) J. E. M. N. Klein and B. Plietker, Org. Biomol. Chem., 2013, 11, 1271 RSC; (l) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (m) M. Darwish and M. Wills, Catal. Sci. Technol., 2012, 2, 243 RSC; (n) O. García Mancheño, Angew. Chem., Int. Ed., 2011, 50, 2216 CrossRef PubMed; (o) A. Schäfer, T. Jurca, J. Turner, J. R. Vance, K. Lee, V. A. Du, M. F. Haddow, G. R. Whittell and I. Manners, Angew. Chem., Int. Ed., 2015, 54, 4836 CrossRef PubMed; (p) M. D. Greenhalgh, A. S. Jones and S. P. Thomas, ChemCatChem, 2015, 7, 190 CrossRef CAS PubMed.
- (a) E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS PubMed , and references therein; (b) S. K. Ghorai, M. Jin, T. Hatakeyama and M. Nakamura, Org. Lett., 2012, 14, 1066 CrossRef CAS PubMed , and references therein.
- (a) R. B. Bedford, M. Huwe and M. C. Wilkinson, Chem. Commun., 2009, 600 RSC; (b) R. B. Bedford, M. A. Hall, G. R. Hodges, M. Huwe and M. C. Wilkinson, Chem. Commun., 2009, 6430 RSC; (c) C. J. Adams, R. B. Bedford, E. Carter, N. J. Gower, M. F. Haddow, J. N. Harvey, M. Huwe, M. Á. Cartes, S. M. Mansell, C. Mendoza, D. M. Murphy, E. C. Neeve and J. Nunn, J. Am. Chem. Soc., 2012, 134, 10333 CrossRef CAS PubMed; (d) R. B. Bedford, E. Carter, P. M. Cogswell, N. J. Gower, M. F. Haddow, J. N. Harvey, D. M. Murphy, E. C. Neeve and J. Nunn, Angew. Chem., Int. Ed., 2013, 52, 1285 CrossRef CAS PubMed; (e) R. B. Bedford, P. B. Brenner, E. Carter, P. M. Cogswell, M. F. Haddow, J. N. Harvey, D. M. Murphy, J. Nunn and C. H. Woodall, Angew. Chem., Int. Ed., 2014, 53, 1804 CrossRef CAS PubMed.
- (a) M. Nakamura, S. Ito, K. Matsuo and E. Nakamura, Synlett, 2005, 11, 1794 CrossRef; (b) T. Hatakeyama, Y. Kondo, Y.-i. Fujiwara, H. Takaya, S. Ito, E. Nakamura and M. Nakamura, Chem. Commun., 2009, 1216 RSC; (c) T. Hatakeyama, N. Nakagawa and M. Nakamura, Org. Lett., 2009, 11, 4496 CrossRef CAS PubMed; (d) S. Kawamura, K. Ishizuka, H. Takaya and M. Nakamura, Chem. Commun., 2010, 46, 6054 RSC; (e) T. Hatakeyama, T. Hashimoto, Y. Kondo, Y. Fujiwara, H. Seike, H. Takaya, Y. Tamada, T. Ono and M. Nakamura, J. Am. Chem. Soc., 2010, 132, 10674 CrossRef CAS PubMed; (f) T. Hatakeyama, T. Hashimoto, K. K. A. D. S. Kathriarachchi, T. Zenmyo, H. Seike and M. Nakamura, Angew. Chem., Int. Ed., 2012, 51, 8834 CrossRef CAS PubMed.
- C. A. Brown, T. A. Nile, M. F. Mahon and R. L. Webster, Dalton Trans., 2015, 44, 12189 RSC.
- R. B. Bedford, P. B. Brenner, E. Carter, J. Clifton, P. M. Cogswell, N. J. Gower, M. F. Haddow, J. N. Harvey, J. A. Kehl, D. M. Murphy, E. C. Neeve, M. L. Neidig, J. Nunn, B. E. R. Snyder and J. Taylor, Organometallics, 2014, 33, 5767 CrossRef CAS.
- R. B. Bedford, Acc. Chem. Res., 2015, 48, 1485 CrossRef CAS PubMed , and references therein.
- (a) M. J. Overett, K. Blann, A. Bollmann, R. de Villiers, J. T. Dixon, E. Killian, M. C. Maumela, H. Maumela, D. S. McGuinness, D. H. Morgan, A. Rucklidge and A. M. Z. Slawin, J. Mol. Catal. A: Chem., 2008, 283, 114 CrossRef CAS PubMed; (b) D. A. Culkin and J. F. Hartwig, J. Am. Chem. Soc., 2001, 123, 5816 CrossRef CAS; (c) C. Daguenet, R. Scopelliti and P. J. Dyson, Organometallics, 2004, 23, 4849 CrossRef CAS; (d) W. You and M. K. Brown, J. Am. Chem. Soc., 2014, 136, 14730 CrossRef CAS PubMed.
- (a) R. Kadyrov, R. M. Koenigs, C. Brinkmann, D. Voigtlaender and M. Rueping, Angew. Chem., Int. Ed., 2009, 48, 7556 CrossRef CAS PubMed; (b) R. Takise, K. Muto, J. Yamaguchi, K. Itami and A. Ignatiev, Angew. Chem., Int. Ed., 2014, 53, 6791 CrossRef CAS PubMed; (c) E. Koch, R. Takise, A. Studer, J. Yamaguchi and K. Itami, Chem. Commun., 2015, 51, 855 RSC; (d) T. Benincori, T. Pilati, S. Rizzo, F. Sannicolò, M. J. Burk, L. de Ferra, E. Ullucci and O. Piccolo, J. Org. Chem., 2005, 70, 5436 CrossRef CAS PubMed.
- L. Zhang, N. S. Colella, B. P. Cherniawski, S. C. B. Mannsfeld and A. L. Briseno, ACS Appl. Mater. Interfaces, 2014, 6, 5327 CAS.
- (a) V. I. Sorokin, M. Nieuwenhuyzen and G. C. Saunders, Mendeleev Commun., 2006, 16, 171 CrossRef PubMed; (b) A. García, J. Gómez, E. Lalinde and M. T. Moreno, Organometallics, 2006, 25, 3926 CrossRef; (c) M. A. Bennett, C. J. Cobley, A. D. Rae, E. Wenger and A. C. Willis, Organometallics, 2000, 19, 1522 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: synthesis and characterisation of the ligands and the method used for the catalytic runs. See DOI: 10.1039/c5cy00851d |
| This journal is © The Royal Society of Chemistry 2015 |