Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
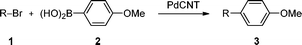
Room temperature Suzuki coupling of aryl iodides, bromides, and chlorides using a heterogeneous carbon nanotube-palladium nanohybrid catalyst†
Dhanaji V.
Jawale
a,
Edmond
Gravel
a,
Caroline
Boudet
a,
Nimesh
Shah
b,
Valérie
Geertsen
c,
Haiyan
Li
d,
Irishi N. N.
Namboothiri
*b and
Eric
Doris
*a
aCEA, iBiTecS, Service de Chimie Bioorganique et de Marquage, 91191 Gif-sur-Yvette, France. E-mail: eric.doris@cea.fr
bDepartment of Chemistry, Indian Institute of Technology Bombay, Mumbai 400 076, India. E-mail: irishi@chem.iitb.ac.in
cCEA, IRAMIS, Nanosciences et Innovation pour les Matériaux, la Biomédecine et l'Energie, UMR3299, 91191 Gif-sur-Yvette, France
dState Key Laboratory of Physical Chemistry for Solid Surfaces and National Engineering Laboratory for Green Chemical Productions of Alcohols, Ethers, and Esters, Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
First published on 29th January 2015
Abstract
Palladium nanoparticles were immobilized on multi-walled carbon nanotubes by a layer-by-layer approach, resulting in a well-defined assembly. The nanohybrid was found effective in promoting Suzuki cross couplings of various halogenated aromatics, including chlorinated ones, with arylboronic acids under sustainable conditions. The heterogeneous catalyst could also easily be recovered from the reaction mixture and reused with no loss of activity over several cycles.
Introduction
The field of organometallic chemistry has expanded spectacularly over the past decades with several Nobel prizes awarded to scientists for their contribution to the domain.1 In particular, the development of palladium-catalyzed cross-coupling reactions has led to advances in synthetic organic chemistry. For example, the Suzuki reaction2 plays a pivotal role in the synthesis of a wide range of compounds, from pharmaceuticals3 to polymers and materials.4 This reaction is classically carried out using Pd0 complexes but heterogeneous Pd-based catalysts have also been developed to allow the recovery and reuse of the precious metal.5 However, most of the supported systems still require organic solvents, additives and heating to be operative. In addition, aryl chlorides are highly challenging substrates as they are known to be poorly reactive under heterogeneous conditions. From these considerations, it appears that the development of a recyclable and broadly active catalyst remains an important issue.6Among the different catalyst supports, nanostructured carbon allotropes, in particular carbon nanotubes (CNTs), have recently emerged as promising materials.7 However, the reported systems all require elevated temperatures and/or high metal catalyst loading, and most notably, none of them address the issue of the Suzuki coupling of aryl chlorides under mild conditions. We previously described the construction and use of nanohybrids made by the assembly of gold or ruthenium nanoparticles on carbon nanotubes.8 In this communication, we report a novel hybrid structure made of supported palladium nanoparticles (PdNPs) on CNTs and its use in the catalysis of the Suzuki cross-coupling reaction under mild conditions.
Results and discussion
Synthesis of the hybrid
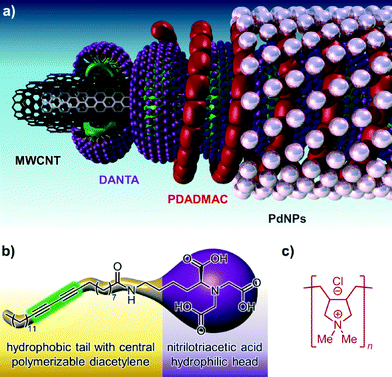
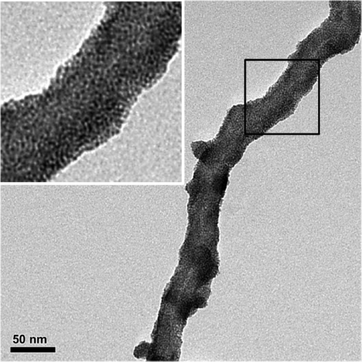
The CNT-supported palladium catalyst (PdCNT) was prepared by a layer-by-layer approach (Fig. 1a) according to a procedure adapted from our previous work.8b The first step consisted in the aqueous self-assembly of amphiphilic nitrilotriacetic-diacetylene lipid (DANTA, Fig. 1b) to form hemi-micelles on multi-walled carbon nanotubes.9 This assembly step gave rise to nanoring-like structures10,11 with the hydrophobic portion of DANTA adsorbed by van der Waals interactions on the nanotube surface and its hydrophilic head oriented outward, at the interface with the aqueous medium.11a Extra stability of the rings was achieved through photo-polymerization of the diyne motif incorporated in the lipophilic chain by UV irradiation at 254 nm. The polymerization process reinforces the cohesion of the supra-molecular assembly around the nanotube.12 The coated nanotubes were then stirred with cationic poly(diallyldimethylammonium chloride) (PDADMAC, Fig. 1c). This step permitted the adsorption of the second layer by electrostatic interactions with the anionic DANTA rings. Finally, freshly prepared PdNPs13 were added to the doubly-coated CNT where the polyammonium network provided robust anchoring and stabilization of the palladium nanoparticles.The nanohybrids were found to be densely covered with Pd nanoparticles. Observation by transmission electron microscopy (TEM) indicated that the PdNPs were of spherical shape and size evaluation by statistical diameter measurement gave a mean particle diameter of ca. 2 nm (Fig. 2). The volume of the aqueous PdCNT suspension was adjusted to a Pd concentration of 6 mM (as determined by ICP-MS). X-ray photoelectron spectroscopy (XPS) analysis indicated that the particles were composed of a mixture of palladium metal and palladium oxide (ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, see ESI,† Fig. S1).
1 ratio, see ESI,† Fig. S1).
Catalytic properties of the PdCNT
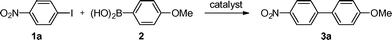
Preliminary studies on the catalytic activity of the PdCNT nanohybrid for the Suzuki reaction were conducted using 4-nitroiodobenzene (1a) and 4-methoxyphenylboronic acid (2) as model partner substrates (Table 1). The reaction was run under ambient conditions (air, room temperature) with 1.2 mol% of PdCNT, K2CO3 as a base, in different solvents. When THF was used, very little conversion was observed even after 24 h (entry 1). In water, the conversion was also limited (6%, 24 h, entry 2), likely because of the poor solubility of the substrates. The best medium was found to be a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ethanol/water which permitted nearly full conversion within 4 h at room temperature (entry 3). Under such conditions, EtOH allows solubility of the substrates while water ensures dispersion of the catalyst, leading to higher overall activity. When PdCNT was replaced by unsupported colloidal PdNPs, only partial coupling (15%) was observed after 4 h (entry 4). No reaction was detected with PdCl2(CH3CN)2 (precursor to the PdNPs), even after 24 h (entry 5). The same comment holds true for the hybrid assembly devoid of PdNPs (i.e. CNTs covered with DANTA rings and PDADMAC) which was found to be totally ineffective (entry 6). These experiments highlight the superior efficacy of the CNT/palladium assembly which can be ascribed to the intrinsic nature of the nanotubes. Indeed, in addition to Pd nanoparticles stabilization by the polyammonium coating, stabilization of transient higher oxidation states of Pd (Pd0/PdII catalytic cycle) by the electronically active nanotubes is also likely operative.14
1 mixture of ethanol/water which permitted nearly full conversion within 4 h at room temperature (entry 3). Under such conditions, EtOH allows solubility of the substrates while water ensures dispersion of the catalyst, leading to higher overall activity. When PdCNT was replaced by unsupported colloidal PdNPs, only partial coupling (15%) was observed after 4 h (entry 4). No reaction was detected with PdCl2(CH3CN)2 (precursor to the PdNPs), even after 24 h (entry 5). The same comment holds true for the hybrid assembly devoid of PdNPs (i.e. CNTs covered with DANTA rings and PDADMAC) which was found to be totally ineffective (entry 6). These experiments highlight the superior efficacy of the CNT/palladium assembly which can be ascribed to the intrinsic nature of the nanotubes. Indeed, in addition to Pd nanoparticles stabilization by the polyammonium coating, stabilization of transient higher oxidation states of Pd (Pd0/PdII catalytic cycle) by the electronically active nanotubes is also likely operative.14
| Entry | Solvent | Catalyst | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Conditions: 1a (0.05 mmol), 2 (0.06 mmol), K2CO3 (2 equiv.), Pd catalyst (1.2 mol%), EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 mL), room temperature, under air.
b Yield of isolated product.
c No reaction. 1, 3 mL), room temperature, under air.
b Yield of isolated product.
c No reaction.
|
||||
| 1 | THF | PdCNT | 24 | 18 |
| 2 | H2O | PdCNT | 24 | 6 |
| 3 | EtOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PdCNT | 4 | 98 |
| 4 | EtOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PdNPs | 4 | 15 |
| 5 | EtOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PdCl2(CH3CN)2 | 24 | NRc |
| 6 | EtOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CNT/DANTA/PDADAMAC | 24 | NRc |
To confirm that the catalytic activity of the PdCNT nanohybrid is due to immobilized PdNPs on CNTs and not to the leaching of Pd in solution, a coupling experiment between 1a and 2 was conducted under the above optimized conditions. After 1 h of reaction, the catalyst was filtered-off. At this point, 1H-NMR analysis showed 31% of the biaryl compound 3a in the crude mixture. Further stirring of the filtrate (devoid of the nanohybrid) for 24 h led to no extra conversion, thus validating the heterogeneous nature of the catalysis.
Recyclability of the hybrid was investigated through sequential couplings of 1a and 2 using the same PdCNT catalyst sample. Briefly, a standard coupling experiment was set-up and after 4 h of reaction, the mixture was centrifuged, the liquid phase was collected, worked-up and the crude product was purified by column chromatography. In parallel, the catalyst-containing pellet was collected and reused for the coupling of fresh substrates 1a and 2. This was done over five consecutive cycles with no drop in catalytic activity (97–98% yield for each run). TEM analysis of the catalyst recovered after 5 cycles showed no major changes in the morphology of the hybrid (Fig. S2a, ESI†). However, XPS analysis indicated that the recovered catalyst was mainly made of Pd metal (Fig. S2b, ESI†), indicating in situ reduction of the Pd oxide fraction during the catalytic cycle.
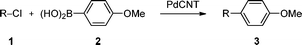
Versatility of the nanohybrid catalyst was demonstrated by coupling 4-methoxyphenylboronic acid (2) with iodinated, brominated, and chlorinated benzenes. In addition to halobenzenes bearing an electron withdrawing group (4-nitrohalobenzenes, 1a-a′′), electron donating- (4-methoxyhalobenzenes, 1b-b′′) and plain halobenzenes (1c-c′′) were also selected for this preliminary screening (Table 2).
| Entry | R | X (1) | Product 3 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
a Conditions: 1 (0.05 mmol), 2 (0.06 mmol), K2CO3 (2 equiv.), PdCNT 6 mM aqueous suspension (100 μL, 1.2 mol%), EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 mL), room temperature, under air.
b Yield of isolated product.
c Reaction carried-out with 2.4 mol% of the catalyst. 1, 3 mL), room temperature, under air.
b Yield of isolated product.
c Reaction carried-out with 2.4 mol% of the catalyst.
|
|||||
| 1 | I (1a) |
 3a
3a
|
4 | 98 | |
| 2 | NO2 | Br (1a′) | 7 | 98 | |
| 3c | Cl (1a′′) | 16 | 95 | ||
| 4 | I (1b) |
 3b
3b
|
8 | 92 | |
| 5 | OMe | Br (1b′) | 11 | 96 | |
| 6c | Cl (1b′′) | 24 | 85 | ||
| 7 | I (1c) |
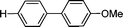 3c
3c
|
5 | 95 | |
| 8 | H | Br (1c′) | 7.5 | 97 | |
| 9c | Cl (1c′′) | 24 | 94 | ||
This first set of experiments showed that, although the catalytic system is efficient with various “R” groups on the halobenzene substrate, the electronic character of the substituent has a marked impact on the kinetics of the reactions. In fact, the formation of the nitro biaryl 3a (entries 1–3) is approximately twice as fast as that of the methoxy analog 3b (entries 4–6). This comment holds true whatever the nature of the halogen atom borne by 1. PdCNT smoothly promotes the coupling of iodinated or brominated aryl substrates and, upon slight increase of the catalytic loading (2.4 mol%) and reaction time, is also efficient for the coupling of chlorinated compounds (entries 3, 6, and 9). It is worth noting that the latter substrates have the reputation of being poorly reactive under heterogeneous coupling conditions.15
The scope of the nanohybrid-promoted reaction was thus further explored by studying the coupling of 4-methoxyphenylboronic acid 2 with a range of brominated (Table 3) and chlorinated substrates (Table 4). Electron withdrawing substituted benzene derivatives such as 4-bromobenzaldehyde (1d′, entry 1), 4-bromoacetophenone (1e′, entry 2), and 4-cyanobromobenzene (1f′, entry 3) were converted in excellent yields to the corresponding biphenyl products 3d (97%), 3e (90%), and 3f (99%) in approximately 7 h (Table 3). The simultaneous double-coupling of 2 on 1,3-dibromobenzene (1g′, entry 4) provided triaryl compound 3g in 98% yield after only 4 h. The activity of the PdCNT catalytic system was also investigated on several nitrogen-, oxygen-, and sulfur-containing heteroaromatic substrates.
| Entry | Substrate 1 | Product 3 | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Conditions: 1 (0.05 mmol), 2 (0.06 mmol), K2CO3 (2 equiv.), PdCNT 6 mM aqueous suspension (100 μL, 1.2 mol%), EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 mL), room temperature, under air.
b Yield of isolated product.
c Reaction carried out with twice the amount of 2 (0.12 mmol).
d No reaction. 1, 3 mL), room temperature, under air.
b Yield of isolated product.
c Reaction carried out with twice the amount of 2 (0.12 mmol).
d No reaction.
|
||||
| 1 |
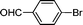 1d′
1d′
|
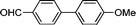 3d
3d
|
7 | 97 |
| 2 |
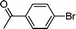 1e′
1e′
|
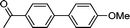 3e
3e
|
6.5 | 90 |
| 3 |
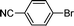 1f′
1f′
|
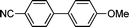 3f
3f
|
7 | 99 |
| 4c |
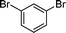 1g′
1g′
|
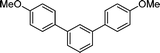 3g
3g
|
4 | 98 |
| 5 |
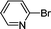 1h′
1h′
|
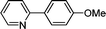 3h
3h
|
6 | 98 |
| 6 |
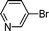 1i′
1i′
|
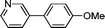 3i
3i
|
24 | 48 |
| 7 |
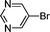 1j′
1j′
|
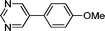 3j
3j
|
24 | 80 |
| 8 |
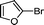 1k′
1k′
|
 3k
3k
|
16 | 97 |
| 9 |
 1l′
1l′
|
 3l
3l
|
15 | 85 |
| 10 |
 1m′
1m′
|
— | 24 | NRd |
| 11 |
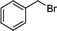 1n′
1n′
|
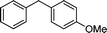 3n
3n
|
48 | 54 |
| Entry | Substrate 1 | Product 3 | Yieldb (%) |
|---|---|---|---|
a Conditions: 1 (0.05 mmol), 2 (0.06 mmol), K2CO3 (2 equiv.), PdCNT 6 mm aqueous suspension (200 μL, 2.4 mol%), EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 mL), room temperature, under air, 24 h.
b Yield of isolated product. 1, 3 mL), room temperature, under air, 24 h.
b Yield of isolated product.
|
|||
| 1 |
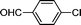 1d′′
1d′′
|
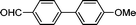 3d
3d
|
85 |
| 2 |
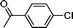 1e′′
1e′′
|
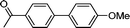 3e
3e
|
86 |
| 3 |
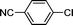 1f′′
1f′′
|
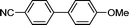 3f
3f
|
89 |
| 4 |
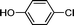 1o′′
1o′′
|
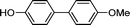 3o
3o
|
75 |
| 5 |
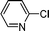 1h′′
1h′′
|
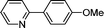 3h
3h
|
87 |
We next investigated the scope of the more challenging PdCNT-mediated Suzuki coupling of chlorinated substrates (Table 4) for which our nanohybrid system proved again to be very efficient. Although slightly higher catalyst loading (2.4 mol%) and reaction time (24 h) were required, the reaction worked equally well on electron deficient (entries 1–3), electron rich (entry 4) and heteroaromatic (entry 5) substrates. These data have to be compared to those of other supported nanocatalysts5 applied to chlorinated substrates which usually require much more drastic conditions (e.g. extensive heating) to be functioning in satisfactory yields.
Conclusions
Carbon nanotubes have been used as support for a new palladium-based heterogeneous catalyst. The PdCNT nanohybrid was used in the promotion of Suzuki cross coupling of iodinated, brominated and chlorinated aromatic substrates with phenylboronic acids. The system proved effective on a variety of substrates including substituted benzenes, heteroaromatics, and even non-aromatic benzyl bromide. The results obtained compare favorably to previous reports7 as the catalyst efficiently operates in green solvents16 (EtOH/H2O mixture), without the need of a controlled atmosphere, and most importantly at room temperature even for chlorinated substrates which is, to the best of our knowledge, unprecedented for heterogeneous catalytic systems.Acknowledgements
Support from the Indo-French Centre for the Promotion of Advanced Research (IFCPAR) / Centre Franco-Indien pour la Promotion de la Recherche Avancée (CEFIPRA) is gratefully acknowledged (Project no.4705-1). The TEM-team platform (CEA, iBiTec-S) is acknowledged for help with TEM images. The “Service de Chimie Bioorganique et de Marquage” belongs to the Laboratory of Excellence in Research on Medication and Innovative Therapeutics (ANR-10-LABX-0033-LERMIT).Notes and references
- C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177 CrossRef CAS PubMed.
- A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577 RSC.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181 RSC.
- M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet and F.-X. Felpin, Adv. Synth. Catal., 2010, 352, 33 CrossRef CAS.
- (a) J. John, E. Gravel, I. N. N. Namboothiri and E. Doris, Nanotechnol. Rev., 2012, 1, 515 CAS; (b) J. Guerra and M. A. Herrero, Nanoscale, 2010, 2, 1390 RSC; (c) A. Corma, H. Garcia and A. Leyva, J. Mol. Catal. A: Chem., 2005, 230, 97 CrossRef CAS PubMed; (d) X. Chen, Y. Hou, H. Wang, Y. Cao and J. He, J. Phys. Chem. C, 2008, 112, 8172 CrossRef CAS; (e) S. Mahouche Chergui, A. Ledebt, F. Mammeri, F. Herbst, B. Carbonnier, H. Ben Romdhane, M. Delamar and M. M. Chehimi, Langmuir, 2010, 26, 16115 CrossRef CAS PubMed; (f) J. A. Sullivan, K. A. Flanagan and H. Hain, Catal. Today, 2009, 145, 108 CrossRef CAS PubMed; (g) N. Karousis, G.-E. Tsotsou, F. Evangelista, P. Rudolf, N. Ragoussis and N. Tagmatarchis, J. Phys. Chem. C, 2008, 112, 13463 CrossRef CAS; (h) M. Cano, A. Benito, W. K. Maser and E. P. Urriolabeitia, Carbon, 2011, 49, 652 CrossRef CAS PubMed; (i) A. R. Siamaki, Y. Lin, K. Woodberry, J. W. Connell and B. F. Gupton, J. Mater. Chem. A, 2013, 1, 12909 RSC.
- (a) E. Gravel, S. Foillard, H. B. Zhang, H. Y. Li and E. Doris, Sci. China: Chem., 2010, 53, 2015 CrossRef CAS; (b) J. John, E. Gravel, A. Hagège, H. Li, T. Gacoin and E. Doris, Angew. Chem., Int. Ed., 2011, 50, 7533 CrossRef CAS PubMed; (c) R. Kumar, E. Gravel, A. Hagège, H. Li, D. V. Jawale, D. Verma, I. N. N. Namboothiri and E. Doris, Nanoscale, 2013, 5, 6491 RSC; (d) R. Kumar, E. Gravel, A. Hagège, H. Li, D. Verma, I. N. N. Namboothiri and E. Doris, ChemCatChem, 2013, 5, 3571 CrossRef CAS; (e) D. V. Jawale, E. Gravel, V. Geertsen, H. Li, N. Shah, I. N. N. Namboothiri and E. Doris, ChemCatChem, 2014, 6, 719 CrossRef CAS; (f) D. V. Jawale, E. Gravel, V. Geertsen, H. Li, N. Shah, R. Kumar, J. John, I. N. N. Namboothiri and E. Doris, Tetrahedron, 2014, 70, 6140 CrossRef CAS PubMed; (g) N. Shah, E. Gravel, D. V. Jawale, E. Doris and I. N. N. Namboothiri, ChemCatChem, 2014, 6, 2201 CrossRef CAS; (h) N. Shah, E. Gravel, D. V. Jawale, E. Doris and I. N. N. Namboothiri, ChemCatChem, 2015, 7, 57 CrossRef CAS; (i) D. V. Jawale, E. Gravel, C. Boudet, N. Shah, V. Geertsen, H. Li, I. N. N. Namboothiri and E. Doris, Chem. Commun., 2015, 51, 1739 RSC.
- P. Chen, H. B. Zhang, G. D. Lin, Q. Hong and K. R. Tsai, Carbon, 1997, 35, 1495 CrossRef CAS.
- (a) C. Thauvin, S. Rickling, P. Schultz, H. Celia, S. Meunier and C. Mioskowski, Nat. Nanotechnol., 2008, 3, 743 CrossRef CAS PubMed; (b) E. Contal, A. Morère, C. Thauvin, A. Perino, S. Meunier, C. Mioskowski and A. Wagner, J. Phys. Chem. B, 2010, 114, 5718 CrossRef CAS PubMed.
- (a) C. Richard, F. Balavoine, P. Schultz, T. W. Ebbesen and C. Mioskowski, Science, 2003, 300, 775 CrossRef CAS PubMed; (b) N. Mackiewicz, G. Surendran, H. Remita, B. Keita, G. Zhang, L. Nadjo, A. Hagège, E. Doris and C. Mioskowski, J. Am. Chem. Soc., 2008, 130, 8110 CrossRef CAS PubMed; (c) N. Khiar, M. P. Leal, R. Baati, C. Ruhlmann, C. Mioskowski, P. Schultz and I. Fernandez, Chem. Commun., 2009, 4121 RSC.
- (a) D. J. Ahn and J.-M. Kim, Acc. Chem. Res., 2008, 41, 805 CrossRef CAS PubMed; (b) Y. Okawa and M. Aono, J. Chem. Phys., 2001, 115, 2317 CrossRef CAS PubMed.
- Palladium particles were synthesized following a procedure inspired by: I. Quiros, M. Yamada, K. Kubo, J. Mizutani, M. Kurihara and H. Nishihara, Langmuir, 2002, 18, 1413 CrossRef CAS.
- (a) G. M. A. Rahman, D. M. Guldi, E. Zambon, L. Pasquato, N. Tagmatarchis and M. Prato, Small, 2005, 1, 527 CrossRef CAS PubMed; (b) M. Melle-Franco, M. Marcaccio, D. Paolucci, F. Paolucci, V. Georgakilas, D. M. Guldi, M. Prato and F. Zerbetto, J. Am. Chem. Soc., 2004, 126, 1646 CrossRef CAS PubMed.
- V. Polshettiwar, A. Decottignies, C. Len and A. Fihri, ChemSusChem, 2010, 3, 502 CrossRef CAS PubMed.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data. See DOI: 10.1039/c4cy01680g |
| This journal is © The Royal Society of Chemistry 2015 |