Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective telomerisation of isoprene with methanol by a heterogeneous palladium resin catalyst
Laura
Torrente-Murciano
*a,
David
Nielsen
b,
Ralf
Jackstell
c,
Matthias
Beller
c,
Kingsley
Cavell
b and
Alexei A.
Lapkin
*d
aDepartment of Chemical Engineering, University of Bath, Bath BA2 7AY, UK. E-mail: ltm20@bath.ac.uk
bDepartment of Chemistry, University of Cardiff, Park Place, Cardiff CF10 3AT, Wales, UK
cLeibniz-Institut für Organische Katalyse an der Universität Rostock e.V., Buchbinderstraße 5-6, 18055 Rostock, Germany
dDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge CB2 3RA, UK. E-mail: aal35@cam.ac.uk
First published on 4th November 2014
Abstract
High catalytic activity for the telomerisation of isoprene with methanol with an unusual regioselectivity towards tail-to-tail telomerisation products was achieved with a heterogeneous (DVB-resin-PPh3-Pd-dvds) catalyst synthesised by supporting palladium-(dvds) on a commercial triphenylphosphine–divinylbenzene resin, with potential for continuous operation. The high activity is a function of the presence of the (dvds) ligand while the regioselectivity is attributed to the combination of (dvds) and PPh3 ligands associated with the palladium centre. The loss of activity upon recycling the catalyst is due to the loss of associated (dvds) ligand during reaction and during the recycle process.
Introduction
Dimerisation of dienes with a nucleophile, known as telomerisation, is a well-studied reaction with a significant synthetic potential.1–5 However, thus far there are few commercial realisations, notably the Kuraray process of telomerisation of butadiene with water with consecutive double bond reduction to yield a linear 1-octanol6 and the Dow Chemical Company telomerisation of butadiene with methanol to produce methoxyocta-2,7-diene which is then reduced to 1-octene.5 The synthetic potential of telomerisation reaction is exemplified by the wide range of nucleophiles shown to be active in telomerisation, such as alcohols,7–10 amines and ammonia,11–19 sugars20–23 and polyols,24–26 carbon dioxide,27etc. It was also shown that longer-chain conjugated dienes could be telomerised28 and internal long-chain dienes could be converted in tandem isomerisation–telomerisation reactions.29 Recent advances in catalyst development, especially the introduction of highly active carbene catalysts with the means of controlling the selectivity between dimerisation and telomerisation products, see e.g.,7,30 should promote a variety of novel applications.The feasibility of developing heterogeneous telomerisation reactions, with a considerably simplified work-up compared to homogeneous single phase catalysis, increases its attractiveness from an industrial point of view. Because of the significant difference in molecular weight of reactants and products, and the possibility of using aqueous solutions of catalysts, the telomerisation reaction can be readily implemented as a bi-phasic aqueous/organic reaction, with the catalysts remaining in the aqueous phase.4,6,17 A number of transition metal catalysed bi-phasic reactions involving decantation as the main separation stage have been successfully commercialised on a large scale, most notably the SHOP process, see e.g., ref. 31. In addition to aqueous phase, catalysts may also be immobilised in ionic liquids (IL), leading to the organic-IL biphasic systems.15,32
Gas–liquid telomerisation represents another type of heterogeneity; telomerisation of gaseous butadiene with liquid acetic acid33 and of gaseous carbon dioxide with liquid butadiene27 have been demonstrated. In the latter case the use of a cyclic carbonate solvent was advantageous over acetonitrile in terms of catalyst activity and selectivity. However, the high boiling point of carbonates does not allow easy product separation by flash distillation, which is a general problem of high-boiling point solvents.
There have been a number of attempts to develop solid heterogeneous telomerisation catalysts. Thus, silica bound Pd(II) (SiO2-acac-Pd(II)) was found to be active in the telomerisation of butadiene with methanol, with a two-fold excess of free alkyl phosphine ligand necessary to ensure selectivity to telomerisation.34 Leaching of Pd was found to be severe, leading to rapid deactivation of the catalysts. A polystyrene-bound Pd(0) triphenylphosphene catalyst was shown to be active in butadiene telomerisation with a number of nucleophiles, but loss of activity was significant already on the second recycle.35 Similar problem of leaching was also observed with Pd(acac)2 catalyst heterogenised on Merrifield resin, where catalysis was due to the leached homogeneous Pd(II) species. However, leaching was found to be much less significant if the Pd(II) or Pd(0) precursor was bound to the resin via a bis(diphenylphosphino)propane or bis(diphenylphosphino)methane ligands. Although the activities of the resulting catalysts were much lower than the homogeneous Pd(II) or Pd(0) catalysts, telomerisation activity appeared to be a product of true heterogeneous catalysis.34,36
The reaction of butadiene with water catalysed by Pd(II) supported on montmorillonite clay showed reasonable activity, with TOF values up to 277 h−1, and was stable to Pd leaching.37 However, in the case of telomerisation with methanol or phenol, the montmorillonite supported Pd(II) catalyst was found to be less stable in recycling experiments than Pd(II) supported on KF/Al2O3 base.38 This is likely to be due to the choice of ligand, rather than the sensitivity of reaction to the nature of the support or the change of nucleophile. The same Pd(TPPTS)n-KF/Al2O3 catalyst was shown to be reasonably active in telomerisation of butadiene with xylose, but showing a 16% loss in activity over five consecutive catalyst recycles.21
More recently the base-free telomerisation system using Pd-TOMP (tris(2-methoxyphenyl)phosphine) catalyst was heterogenised in double layer hydroxides, hydrotalcites.39 Heterogenisation resulted in a significant loss of activity, with 51% conversion in the case of butadiene–methanol system with only 18% selectivity to C8 products, and only two recycles were reported with a significantly lower conversion, which were shown to be due to loss of Pd. Much better activities and catalyst re-usability were achieved in the case of the Pd catalysts immobilised within a covalent organic framework support (abbreviated as COF), although metal Pd particles were observed in the spent catalysts.40
Another approach to heterogenisation is through complexation of the active catalyst in a macrocycle, which leads to the formation of a soluble ‘heterogeneous’ catalyst, recycled by distilling solvent and products. An effective recycling of Pd(0) catalyst was shown in the telomerisation of butadiene with methane.41
There are only few reports of the successful heterogenisation of telomerisation catalysts, mainly due to the complexity of the system. The reactant diene and free ligands are in competition for co-ordination with Pd and thus strong interaction between Pd and the support is required, which may consequently lead to the loss of activity or selectivity. The most successful reported heterogeneous telomerisation catalysts are based on resins where the coordination sphere of the active Pd species are similar to the corresponding molecular homogeneous catalysts; the Pd coordination to the polymer is sufficiently strong to ensure low levels of leaching.5
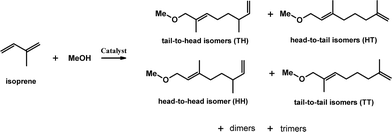
Apart from the expected benefits of recycling the catalyst, the heterogeneous environment may also alter the regioselectivity of the reaction. Telomerisation of isoprene can lead to at least four isomer products shown in Fig. 1, as well as unwanted dimer and trimer by-products.
Out of the telomer products, the head-to-tail isomer would mimic the naturally occurring terpenes, although the main product is typically the tail-to-tail isomer, specially with a Pd-tributylphosphine catalyst.18
In this paper, we report the heterogeneous telomerisation of isoprene with methanol using Pd-dvds (dvds = 1,1,3,3-tetramethyl-1,3-divinyl-disiloxane) on triphenylphosphine–divinylbenzene copolymer. An unusual tail-to-tail telomerisation regioselectivity is reported with this solid catalyst.
Results and discussion
Heterogeneous ResCat 1 (DVB-resin-PPh3-Pd-dvds) and ResCat 2 (DVB-resin-PPh3-Pd) synthesised by attaching palladium complexes to the commercial triphenylphosphine–divinylbenzene copolymer (DVB) resin were studied for the telomerization of isoprene with methanol (see results in Table 1). A series of homogeneous catalysts were also tested under the same conditions for comparison. ResCat 1 showed the best performance, with a conversion of 85% after 7 hours at 70 °C. However, the conversion decreased at a higher temperature, probably due to decomposition of the Pd species immobilised on the resin as we have recently observed in the equivalent homogeneous system.29 The main telomerisation product obtained is the tail-to-tail isomer, which is the less favoured product in homogenous reactions, whether using a carbene (Pd(IMes)(dvds)) or triphenylphosphine as palladium ligand. Interestingly, higher temperatures, >70 °C, favoured the formation of an even greater proportion of tail-to-tail telomerisation isomer although conversion is compromised due to decomposition as previously mentioned. To the best of our knowledge, ResCat 1 shows a higher selectivity for the tail-to-tail product than any previously reported catalyst.| Catalyst | T/°C | TON | Conversion/% @7 h | Pd leachinga/ppm | Selectivityb/% @7 h | Regioselectivity | |||
|---|---|---|---|---|---|---|---|---|---|
| HT | HH | TH | TT | ||||||
| Reaction conditions: 3.25 mL isoprene (4.35 M); solvent: 3.25 mL dry 1% NaOMe in MeOH; 1 mL decane as internal standard. Reaction time = 7 h. Catalyst: 0.025 g of ResCat 1, 0.05 g of ResCat 2, 4 × 10−6 moles for the homogeneous catalysts.a Amount of metal leached into solution determined by atomic absorption.b Selectivity to all telomerisation products. | |||||||||
| ResCat 1 | 70 | 906 | 84.7 | 127 | 58 | 31.6 | 5.7 | 0.9 | 61.8 |
| ResCat 1 | 130 | 206 | 21.0 | Na | 60 | 20.7 | 6.6 | 2.6 | 70.1 |
| ResCat 2 | 70 | 448 | 10.5 | 10 | 17 | 45.2 | 5.2 | 49.6 | — |
| Pd(IMes)(dvds) | 70 | 3694 | 45.5 | — | 88 | 14.2 | 85.0 | 0.8 | — |
| Pd(acac)2 + 3 eq. PPh3 | 70 | 862 | 10.6 | — | 73 | 67.5 | 5.5 | 26.9 | — |
Although some leaching of palladium into solution was observed from ResCat 1, the catalytic activity cannot be attributed to the homogeneous leached component, as the regioselectivity of the telomerisation products is not consistent with that obtained with homogeneous catalysts. Furthermore, resins which showed leaching generally exhibit poor catalytic activity, suggesting that the leached palladium species are not catalytically active to a significant degree.
In the case of ResCat 2, conversion was considerably lower compared to ResCat 1. The telomerization product distribution with this heterogeneous catalyst was similar to that observed for the homogeneous Pd(acac)2 in addition of triphenylphosphine ligand. This suggests that the regioselectivity obtained with ResCat 1 is not due to the triphenylphosphine group or the resin itself, but to the combination of the (dvds) and triphenylphosphine ligands associated with the palladium active centre.
The bonding of the palladium complex, (dvds) ligand and the resin backbone was followed by IR. FTIR spectra of the commercial triphenylphosphine DVB-resin, triphenylphosphine and (dvds) ligands are shown for comparison with ResCat 1 and ResCat 2 (Fig. 2). The commercial resin shows the same bands than the PPh3 with the addition of two bands between 2800 and 3100 cm−1 which correspond to DVB resin. There is no apparent distinction between the ResCat 2 and the metal-free resin spectra, which is attributed to the low energy of the Pd–P bonds. Additional bands appear in the ResCat 1 spectrum respect to the commercial resin at 845, 1070 and 1260 cm−1, aligned to those present in the (dvds) spectra, corresponding to the Si–O–Si bending, stretching and asymmetric stretching modes respectively.42 As expected, these bands are not present in the ResCat 2 spectrum.
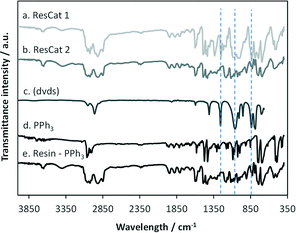 | ||
| Fig. 2 FTIR spectra of a. ResCat 1, b. ResCat 2, c. (dvds) from NIST database n. 2627-95-4, d. PPh3 and e. Commercial triphenylphosphine–divinylbenzene copolymer (DVB) resin. | ||
In order to study the potential for recycling of ResCat 1, consecutive runs using the same catalyst under the same reaction conditions were carried out over a period of several days. After each run, solvent, reactants and products were removed and the ResCat 1 catalyst was stored under solvent (methanol) and recharged the next day with fresh reactants. The conversion and selectivity towards telomerisation products obtained for each run are shown in Fig. 3.
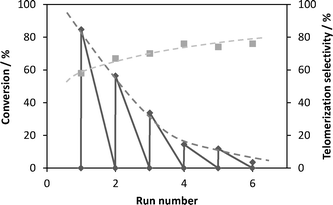 | ||
| Fig. 3 Reaction profile of consecutive telomerisation reactions of isoprene with methanol using ResCat 1 as catalyst. | ||
The decrease in conversion in subsequent runs cannot be explained by simple loss of resin catalyst due to manipulations, as this was estimated at no more than 5% between each run. The overall TON (Table 2) from the catalyst recycle reactions is in the same order of magnitude as those obtained using an equivalent homogeneous catalyst.29 The chemoselectivity and regioselectivity across each run remain largely unchanged, suggesting that the same active palladium species bonded to (dvds) and triphenylphosphine ligands is responsible for the activity across all runs. The loss of (dvds) ligand clearly deactivates the catalysts instead of simply modifying its reactivity.
| Run | Overall TON | Conversion/% @7 h | Pd leachinga/ppm | Selectivityb/% @7 h | Regioselectivity | |||
|---|---|---|---|---|---|---|---|---|
| HT | HH | TH | TT | |||||
| Reaction conditions: 3.25 mL isoprene (4.35 M); solvent: 3.25 mL dry 1% NaOMe in MeOH; 1 mL decane as internal standard. Reaction time = 7 h. Catalyst: 0.025 g of ResCat 1, 0.05 g of ResCat 2, 4 × 10−6 moles for the homogeneous catalysts.a Amount of metal leached into solution determined by atomic absorption.b Selectivity to all telomerisation products. | ||||||||
| 1 | 906 | 84.7 | 127 | 58 | 31.6 | 5.7 | 0.9 | 61.8 |
| 2 | 1510 | 56.4 | 48 | 67 | 28.0 | 5.5 | 1.4 | 65.1 |
| 3 | 1868 | 33.7 | 15 | 70 | 29.0 | 5.4 | 0.4 | 65.1 |
| 4 | 2022 | 14.4 | 11.5 | 76 | 27.8 | 5.1 | 2.4 | 64.7 |
| 5 | 2149 | 11.8 | 5.7 | 74 | 33.6 | 4.9 | 2.6 | 58.9 |
| 6 | 2186 | 3.5 | — | 76 | 34.4 | 5.8 | 3.2 | 56.7 |
The loss of activity upon recycling of the catalyst could be due to erosion of the catalyst from the resin particles, as was claimed in previous heterogeneous telomerisation reactions using polymer supports.43Fig. 4 shows overview SEM images of the fresh ResCat 1 catalyst and the beads recovered after six telomerisation runs. Although some of the particles were ruptured during the Pd immobilisation process, likely due to the effects of mechanical stirring during the synthesis, most of the fresh catalyst beads remained intact. However, the rupture effect is magnified during the telomerisation runs when most of the particles been fractured after six runs.
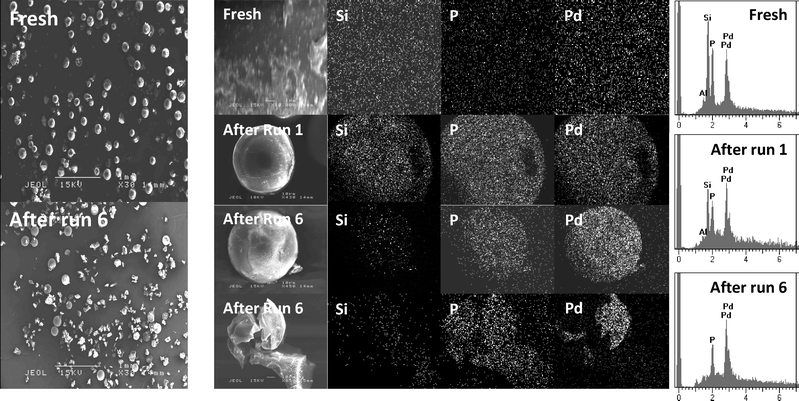 | ||
| Fig. 4 Scanning electron microscopy (SEM) and Energy dispersive X-ray spectroscopy (EDXS) elemental mapping of ResCat 1 catalysts fresh, after one catalytic run and after 6 catalytic runs. | ||
Fig. 4 shows a higher magnification SEM image of a fresh ResCat 1 bead where physical defects on the surface become apparent. However, EDXS elemental mapping shows a homogeneous distribution of palladium, silicon and phosphorous across the bead surface. A similar chemical mapping of the beads after the first telomerisation run reveals that, despite the catalyst becoming black during the reaction, a similar homogeneous distribution of Pd, Si and P across the bead than the fresh catalyst is observed. This suggests that there was little loss of metal or ligands from the surface of ResCat 1 during the telomerisation reaction. It also indicates that there is not an accumulation of palladium nanoparticles on the resin surface. When the same analysis was performed on an unbroken particle of ResCat 1 after six recycling runs, very little Si was observed at the surface, although the Pd and P distribution remained homogeneous. A similar chemical composition is observed in a fractured ResCat 1 bead after six runs. The internal and external surfaces of the resin can be easily distinguished by the X-ray analysis, with the external surface retaining a homogeneous distribution of P and Pd. This suggests that broken resin beads should retain their catalytic activity and that the on-going loss of activity in subsequent runs is a consequence of chemical changes rather than the observed macroscopic physical changes in the beads. The elemental mapping also shows that Pd is not being homogenised and re-deposited on the resin, including the internal surfaces of broken particles, during the telomerisation reactions.
Fig. 4 also shows the elemental analyses of EDX analyses of the fresh ResCat 1 catalyst in comparison to the ones after one and six telomerisation runs. Due to the rounded surface of the resin, these analyses cannot be used quantitatively; however, ratios of elements can be considered. The Pd/P ratio remains constant throughout the recycling runs, but the amount of Si present on the resin decreases after the first run and is almost negligible after six recycling runs. This suggests that loss of the Pd-dvds association is related to catalyst deactivation and is consistent with the low activity shown by ResCat 2, which does not contain (dvds). The low activity of ResCat 1 at 130 °C (Table 1) may also be explained by increased labilisation of the (dvds) ligand at high temperature.
This change in composition of ResCat 1 following recycling is further confirmed by FTIR analysis, Fig. 5. The bands corresponding to Si–O–Si bonds at 845, 1070 and 1260 cm−1 in the spectrum of fresh ResCat 1 decrease in intensity following the first run, and are not present after six recycling runs.
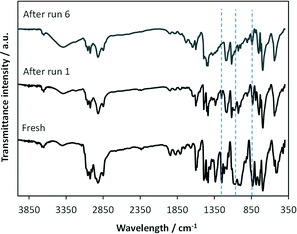 | ||
| Fig. 5 FTIR spectra of the ResCat 1 catalysts: fresh, after one and after six telomerisation recycling runs. | ||
Experimental
Commercial triphenylphosphine–divinylbenzene copolymer (DVB) resin (Fisher Chemicals) was used as heterogeneous support. For the synthesis of resin catalyst 1 (ResCat 1), 1.94 g of the commercial triphenylphosphine resin, (1% DVB, 1–1.5 mmol (PPh3) g−1, 100–200 mesh) was stirred overnight with 3.25 mL of Pd-dvds solution (2.43 mmol Pd) in dry THF, under nitrogen (Fig. 6a). The resin was then filtered and washed with a total of 40 mL of dry THF before drying briefly under vacuum. The solution remaining after separation of the resin was analysed by atomic absorption spectroscopy (Perkin Elmer), indicating that 96.6% of the palladium was adsorbed, resulting in a concentration of 12.9 wt% Pd on the resin. The ResCat 1 was stable under air at room temperature.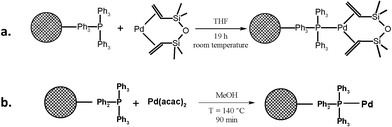 | ||
| Fig. 6 Synthesis of resin bound catalysts a. DVB-resin-PPH3-Pd-dvds (ResCat 1) and b. DVB-resin-PPh3-Pd (ResCat 2). | ||
ResCat 2 was synthesised following a procedure previously described.44 PPh3-resin (0.483 mmol, 1.25 mmol (PPh3) g−1, 0.385 g), Pd(acac)2 (3.4 × 10−3 mmol, 7.25 mg) and 7 mL of MeOH were stirred at 140 °C for 90 minutes in a sealed tube (Fig. 6b). The mixture was cooled to room temperature and the pale yellow solid filtered and washed carefully with methanol. Finally, the catalyst was dried at 120 °C for 2 hours. The palladium loading was determined by inductively coupled plasma analysis (Varian VISTA AX) indicating a concentration of 2.22 wt% Pd on the resin.
The complex (1,3-dimesitylimidazolin-2-ylidene)-palladium(0)-η2,η2-1,1,3,3-tetramethyl-1,3-divinyl-disiloxane (Pd(IMes)(dvds)) was synthesised as previously reported.45
A JEOL JSM-6310 scanning electron microscope was used to obtain images of the morphology and chemical structure of the resin catalysts. The samples to be analysed were attached to a disk using a carbon-based paste and coated with a fine layer of carbon to enhance conductivity before analysis. Infrared spectra were obtained using an EQUINOX 55 spectrometer with a DTGS detector. Sample pellets consisted of 0.2 g of KBr and a pre-determined quantity of sample.
Telomerization reactions were carried out using 15 mL Ace Pressure Tubes equipped with a FETFE® O-ring seated under the rim of a PTFE bushing, supplied by Sigma Aldrich. A heater plate controlled by a Pt100 sensor was used to maintain reaction temperature within ±0.1 °C. The oil bath was pre-heated for 40 minutes before starting a reaction. In a typical experiment, catalyst, solvent, reactant and stirrer bar were introduced into the tube, which was then flushed with nitrogen to remove oxygen. An initial sample was taken for analysis. The pressure tube was closed, placed in the oil bath and the reaction time commenced. After the desired reaction time, the tube was immersed in an ice-bath to quench the reaction and condense any vapours. Samples were taken at intermediate and final reaction times and analysed by GC (Varian 3900) using a non-polar capillary column (Alltech, AT-5) and flame ionization detector (FID). Catalysts were recovered by filtration and the palladium content of the remaining solution analysed by AAS. The material balance was closed in all reactions to within ±5%, and the accuracy of concentration measurements as determined by GC was better than ±5%. Conversion and turnover numbers were calculated based on the disappearance of reactant.
Conclusions
DVB-resin-PPh3-Pd-dvds was shown to be a highly active heterogeneous telomerisation catalyst with an unusual regioselectivity to the tail-to-tail product due to the combination of the triphenilphosphine and (dvds) ligands to the palladium active centre. The catalyst can successfully be recycled in consecutive telomerisation runs although a decrease of activity and, in a minor extend, selectivity was observed due to the leaching of the (dvds) ligand. Such catalytic system is particularly attractive to a potential continuous flow heterogeneous process.Acknowledgements
The authors would like to acknowledge the UK Engineering and Physical Sciences Research Council (projects GR/S86105/01 and GR/S86112/01) for funding.Notes and references
- E. J. Smutny, Oligomerization and dimerization of butadiene under homogeneous catalysis. Reaction with nucleophiles and the synthesis of 1,3,7-octatriene, J. Am. Chem. Soc., 1967, 89, 6793–6794 CrossRef CAS.
- S. Takahashi, T. Shibano and N. Hagihara, The dimerization of butadiene by palladium complex catalysts, Tetrahedron Lett., 1967, 8(26), 2451–2453 CrossRef.
- A. Zapf and M. Beller, Fine chemical synthesis with homogeneous palladium catalysts: examples, status and trends, Top. Catal., 2002, 19, 101–109 CrossRef CAS.
- B. Cornils, Bulk and fine chemicals via aqueous biphasic catalysis, J. Mol. Catal. A: Chem., 1999, 143, 1–10 CrossRef CAS.
- A. Behr, et al., Telomerization: Advances and Applications of a Versatile Reaction, Angew. Chem., Int. Ed., 2009, 48(20), 3598–3614 CrossRef CAS PubMed.
- N. Yoshimura, Applied homogeneous catalysis with organometallic compounds, in Applied homogeneous catalysis with organometallic complexes, ed. B. Cornils and W. A. Herrmann, 1996, VCH: Weinheim, Germany, p. 351 Search PubMed.
- R. Jackstell, et al., A highly efficient catalyst for the telomerization of 1,3-dienes with alcohols: first synthesis of a monocarbenepalladium(0)-olefin complex, Angew. Chem., Int. Ed., 2002, 41, 986–989 CrossRef CAS.
- R. Jackstell, et al., Efficient telomerization of 1,3-butadiene with alcohols in the presence of in situ generated palladium(0)carbene complexes, J. Mol. Catal. A: Chem., 2002, 185, 105–112 CrossRef CAS.
- J. Muzart, Palladium-catalysed reactions of alcohols. Part C: Formation of ether linkages, Tetrahedron, 2005, 61, 5955–6008 CrossRef CAS PubMed.
- A. Krotz, et al., Salt-free C–C coupling reactions of arenes: palladium-catalyzed telomerization of phenols, Chem. Commun., 2001, 195–196 RSC.
- A. Grotevendt, et al., Efficient catalysts for telomerization of amines, Tetrahedron Lett., 2007, 48, 9203–9207 CrossRef CAS PubMed.
- S. M. Maddock and M. G. Finn, Palladium-catalyzed head-to-head telomerization of isoprene with amines, Organometallics, 2000, 19, 2684–2689 CrossRef CAS.
- F. Leca and R. Réau, 2-Pyridyl-2-phospholenes: New P,N ligands for the palladium-catalyzed isoprene telomerization, J. Catal., 2006, 238, 425–429 CrossRef CAS PubMed.
- M. S. Viciu, et al., Telomerization of amines mediated by cationic N-heterocyclic carbene (NHC) palladium complexes, Organometallics, 2003, 22, 3175–3177 CrossRef CAS.
- G. S. Fonseca, R. F. D. Souza and J. Dupont, Biphasic telomerization of 1,3-butadiene with HNEt2 catalyzed by palladium/sulphonated–phosphine complexes, Catal. Commun., 2002, 3, 377–380 CrossRef.
- T. Antonsson and C. Moberg, Palladium-catalysed telomerisation of dienes and tertiary allylic amines. A novel reaction involving cleavage of the carbon-nitrogen bond, Organometallics, 1985, 4, 1083–1086 CrossRef CAS.
- T. Prinz and B. Driessen-Hölscher, Biphasic catalyzed telomerization of butadiene and ammonia: kinetics and new ligands for regioselective reactions, Chem. – Eur. J., 1999, 5, 2069–2076 CrossRef CAS.
- W. Keim, K.-R. Kurtz and M. Röper, Palladium catalysed telomerisation of isoprene with secondary amines and conversion of the resulting terpene amines to terpenols, J. Mol. Catal., 1983, 20, 129–138 CrossRef CAS.
- T. Mitsuyasu, M. Hara and J. Tsuji, Synthesis of long-chain amines by palladium-catalysed telomerisation of butadiene, J. Chem. Soc. D, 1971, 345 RSC.
- V. Desvergnes-Breuil, C. Pinel and P. Gallezot, Green approach to substituted carbohydrates: telomerisation of butadiene with sucrose, Green Chem., 2001, 3(4), 175–177 RSC.
- B. Estrine, et al., Recycling in telomerization of butadiene with D-xylose: Pd(TPPTS)n-KF/Al2O3 as an active catalyst, Appl. Organomet. Chem., 2007, 21, 945–946 CrossRef CAS.
- B. Estrine, et al., Telomerization of butadiene with pentoses in water: selective etherifications, Green Chem., 2005, 7, 219–223 RSC.
- F. Hénin, et al., Palladium-catalyzed telomerization of butadiene with tri-O-acetylated pentoses as a convenient route to 2,7-octadienyl glycosides, Eur. J. Org. Chem., 2004, 511–520 CrossRef.
- A. Behr and M. Urschey, Highly selective biphasic telomerization of butadiene with glycols: scope and limitations, Adv. Synth. Catal., 2003, 345, 1242–1246 CrossRef CAS.
- A. Behr and M. Urschey, Palladium-catalyzed telomerization of butadiene with ethylene glycol in liquid single phase and biphasic systems: control of selectivity and catalyst recycling, J. Mol. Catal. A: Chem., 2003, 197, 101–113 CrossRef CAS.
- R. Palkovits, et al., Highly active catalysts for the telomerization of crude glycerol with 1,3-butadiene, ChemSusChem, 2008, 1, 193–196 CrossRef CAS PubMed.
- A. Behr, et al., Application of carbonate solvents in the telomerisation of butadiene with carbon dioxide, J. Mol. Catal. A: Chem., 2007, 267, 149–156 CrossRef CAS PubMed.
- L. Torrente-Murciano, et al., Telomerisation of long chain dienes with alcohols using Pd(Imes)(dvds) catalyst, Green Chem., 2010, 12, 866–869 RSC.
- L. Torrente-Murciano, et al., Tandem isomerization/telomerization of long chain dienes, Front. Chem., 2014, 2, 37 Search PubMed.
- R. Jackstell, et al., An industrially viable catalyst system for palladium-catalyzed telomerizations of 1,3-butadiene with alcohols, Chem. – Eur. J., 2004, 10(16), 3891–3900 CrossRef CAS PubMed.
- D. Vogt, Economical applications (SHOP Process), in Multiphase homogeneous catalysis, ed. B. Cornils, et al., 2005, Wiley-VCH: Weinheim, pp. 330–338 Search PubMed.
- L. Magna, et al., The importance of imidazolium substituents in the use of imidazolium-based room-temperature ionic liquids as solvents for palladium-catalyzed telomerization of butadiene with methanol, Organometallics, 2003, 22, 4418–4425 CrossRef CAS.
- A. Behr, T. Beckmann and P. Schwach, Multiphase telomerisation of butadiene with acetic acid and acetic anhydride, J. Organomet. Chem., 2008, 693, 3097–3102 CrossRef CAS PubMed.
- F. Benvenuti, et al., 1,3-butadiene telomerization with methanol catalyzed by heterogenized palladium complexes, J. Mol. Catal. A: Chem., 1999, 137, 49–63 CrossRef CAS.
- K. Kaneda, et al., Selective telomerization of butadiene with various nucleophiles catalyzed by polymer-bound palladium(0) complexes, J. Org. Chem., 1981, 46(11), 2356–2362 CrossRef CAS.
- F. Benvenuti, et al., 1,3-butadiene telomerization with methanol catalyzed by heterogenized palladium-II/complexes anchored to polymer-bound bis(diphenylphosphino)methane moieties, J. Mol. Catal. A: Chem., 1999, 139, 177–187 CrossRef CAS.
- B. I. Lee, K. H. Lee and J. S. Lee, Telomerization of butadiene with water catalyzed by heterogeneous palladium catalysts, J. Mol. Catal. A: Chem., 2000, 156, 283–287 CrossRef CAS.
- B. Estrine, et al., Recycling in telomerization of butadiene with methanol and phenol: Pd–KF/Al2O3 as an active heterogeneous catalyst system, Green Chem., 2003, 5, 686–689 RSC.
- A. N. Parvulescu, et al., Telomerization of 1,3-Butadiene with Biomass-Derived Alcohols over a Heterogeneous Pd/TPPTS Catalyst Based on Layered Double Hydroxides, ACS Catal., 2011, 1(5), 526–536 CrossRef CAS.
- P. J. C. Hausoul, et al., Development of a 4,4 '-biphenyl/phosphine-based COF for the heterogeneous Pd-catalysed telomerisation of 1,3-butadiene, Catal. Sci. Technol., 2013, 3(10), 2571–2579 CAS.
- B. Estrine, et al., 15-Membered macrocyclic triolefin: role in recovering active palladium catalyst for the telomerization of butadiene with methanol, Tetrahedron Lett., 2001, 42, 7055–7057 CrossRef CAS.
- D. Rajput, et al., Silica coating of polymer nanowires produced via nanoimprint lithography from femtosecond laser machined templates, Nanotechnology, 2012, 23(10), 6 CrossRef PubMed.
- F. Benvenuti, et al., 1,3-butadiene telomerization with methanol catalyzed by heterogenized palladium complexes, J. Mol. Catal. A: Chem., 1999, 137, 49–63 CrossRef CAS.
- V. Ananikov, M. Kabeshov and I. Beletskaya, The first example of polymer-supported palladium catalyst for stereo-selective S-S bond addition to terminal alkynes, Synlett, 2005, 6, 1015–1017 CrossRef.
- R. Jackstell, et al., An industrially viable catalyst system for palladium-catalyzed telomerizations of 1,3-butadiene with alcohols, Chem. – Eur. J., 2004, 10, 3891–3900 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |