Molybdenum blue nano-rings: an effective catalyst for the partial oxidation of cyclohexane†
Xi
Liu
a,
Marco
Conte
ab,
Weihao
Weng
c,
Qian
He
c,
Robert L.
Jenkins
a,
Masashi
Watanabe
c,
David J.
Morgan
 a,
David W.
Knight
a,
Damien M.
Murphy
a,
Keith
Whiston
d,
Christopher J.
Kiely
c and
Graham J.
Hutchings
*a
a,
David W.
Knight
a,
Damien M.
Murphy
a,
Keith
Whiston
d,
Christopher J.
Kiely
c and
Graham J.
Hutchings
*a
aCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK. E-mail: hutch@cardiff.ac.uk
bDepartment of Chemistry, Dainton Building, University of Sheffield, Sheffield, S3 7HF, UK
cDepartment of Materials Science and Engineering, Lehigh University, 5 East Packer Avenue, Bethlehem, PA 18015-3195, USA
dINVISTA Textiles (UK) Limited, P.O. Box 2002, Wilton, Redcar TS10 4XX, UK
First published on 23rd October 2014
Abstract
Molybdenum blue (MB), a multivalent molybdenum oxide with a nano-ring morphology is well-known in analytical chemistry but, to date it has been largely ignored in other applications. In the present work, MB has been characterized by STEM-HAADF imaging for the first time, showing the nano-ring morphology of this complex molybdenum oxide and the ordered super-molecular framework crystals that can result from the self-assembly of these MB nano-ring units. The potential of MB as an oxidation catalyst has also been investigated, where it is shown to have excellent catalytic activity and stability in the selective oxidation of cyclohexane to cyclohexanol and cyclohexanone which are important intermediates in the production of nylon.
1. Introduction
In the present paper, we demonstrate that a material that has been known for centuries, but which until now has been largely ignored for catalyst applications, possesses exciting catalytic properties. The material in question is molybdenum blue (MB)1 which is well-known in colorimetric and quantification assays, particularly for phosphorus.2,3 It was first synthesized over 200 years ago,4 however, its structure has been enigmatic, with the first convincing models of its atomic arrangement emerging in 1995.5 The classic X-ray diffraction studies of MB by Müller et al. unveiled the linkage of [(MoV/MoVI)mO]n units into giant wheel-shaped clusters by hydrogen bonding.5 More recently, Zhong et al. have used scanning tunnelling microscopy (STM) to directly image individual isolated MB rings,6 but to date there have been no direct observations of the MB nano-structure using transmission electron microscopy. MB is considered to be comprised of complex assemblies of wheel-shaped mixed-valence: Mo154 polyoxomolybdate, [MoVI126MoV28O462H14(H2O)70]14− anion clusters.5,7,8 We reasoned that MB nano-ring structures and their complex assemblies should in principle be observable using high angle annular dark field (HAADF) imaging in the scanning transmission electron microscope (STEM). To date there has been limited progress in the characterization of the microstructure of these mixed-valency molybdenum compounds by electron microscopy. Nevertheless, high-resolution TEM images acquired in these previous studies show poor correlation with the STM images obtained by Zhong et al.,6 as no discrete nano-ring structures were observed. While there has been considerable interest in the structure of MB nano-rings and their molecular assemblies, its use as a catalyst has not been considered in any great detail. In this paper, we present the first clear HAADF-STEM images of MB nano-ring structures. In addition, we demonstrate that the [(MoV/MoVI)mO]n units present within the nano-ring structure can be highly active for the catalysis of hydrocarbon oxidation.Selective oxidation is an important process for the functionalization of chemical feedstocks and there is a continual need to identify new catalysts particularly if they demonstrate interesting selectivity to desirable partial oxidation products. Catalysts have been formulated based on mixed oxides and supported metals for a wide range of such reactions.9 There has been particular interest in utilising molybdenum oxide containing catalysts, including the use of heteropolyacids.10–12 In the majority of these reports the molybdenum is usually present as MoVI species. However, it has been demonstrated that an activating pre-treatment step in an H2 or a hydrocarbon atmosphere is very important in establishing the activity and selectivity of oxidized molybdenum carbides.9 It was noted that this type of activation pre-treatment generates some MoV species as well as MoVI which results in materials with strong blue colorations due to electron exchanges between nearby MoV and MoVI cations in the structure.9,13 We also note that in many previous studies of other molybdenum-based materials, including molybdenum sulfides and molybdenum oxides, there have been reports of a strong blue colouration, thereby suggesting the possible formation of MB-like species during their preparation.9–12 Consequently, we reasoned that MB, which exhibits significant electron hopping between MoV and MoVI sites via MoV–O–MoVI bridging bonds, might represent a material that could be highly active for selective redox chemistry.6 In particular, we considered that MB should be active for the oxy-functionalization of alkanes to selective oxidation products such as alcohols, ketones and acids, and additionally that this process should be feasible under mild reaction conditions. Identification of new catalysts for this type of chemistry is of considerable interest to both academia and industry.9,14–20 A key reaction in this class is the selective oxidation of cyclohexane, as this is of great importance as an intermediate step in nylon manufacture which has an annual production of over 3M tonnes and is therefore a reaction of immense practical and commercial significance.18 The current industrial process for cyclohexane oxidation uses radical autoxidation promoted by cobalt ions which significantly impedes selectivity control.14,15,18–21 In this paper, we show that MB can provide a novel catalytic oxygenation of cyclohexane giving high selectivity to partial oxidation products and we discuss this catalysis in relation to the unique structure of this fascinating material.
2. Experimental methods
2.1 Synthesis of molybdenum blue
Molybdenum blue (MB) was prepared via a facile synthesis method. Powdered metallic molybdenum (Sigma, assay 99.99%) was mixed with diluted hydrogen peroxide (Aldrich 30 wt%, trace metals <10 ppm) and afterwards the suspension was stirred overnight at room temperature. After filtration, the blue solution formed was evaporated to dryness using a rotary evaporator at room temperature in order to obtain a fine blue powder. Compared to other preparation protocols for MB,5 the main advantage of the method employed in this work, is that the MB prepared is relatively free from impurities.2.2 Characterization of the catalyst
2.3 Catalyst testing
Standard reaction conditions for the oxidation of cyclohexane were as follows. Cyclohexane (Alfa Aesar, 8.5 g, HPLC grade) was reacted in a glass bench reactor with catalysts (6 mg). To control accurately the amount of the MB catalyst we used, a dilute aqueous solution of MB with a known concentration of MB was prepared. The desired volume of the MB solution was dispensed as a drop into the glass reactor which was then evaporated to dryness at 30 °C. It should be noted that while MB is soluble in water, it is insoluble in cyclohexane and so using our reaction conditions we consider that the molybdenum blue is present as a heterogeneous catalyst. The reaction mixture was magnetically stirred at 140 °C and 3 bar O2 for 17 h. The reaction mixture was analysed by gas chromatography (Varian 3200) with a CP-Wax 42 column. It should also be noted that acid products, such as adipic acid, were converted to their corresponding ester for quantification purposes.22 For a comparison with the industrial process, the oxidation reactions were also performed by using promoters (6 mg) comprising cobalt naphthenate and ferric acetylacetonate, at 140 °C and 3 bar O2 for 17 h.Re-usability testing was also performed in an identical glass reactor. Cyclohexane (8.5 g) and MB (60 mg) were reacted at 140 °C and 3 bar O2 for 17 h. After reaction, the ‘used’ catalyst was washed with cyclohexane and dried. The catalytic activity of the used MB was re-tested under the standard reaction conditions, and the products were analysed by gas chromatography. Subsequent re-usability tests were carried out on the same material using another iteration of the procedure.
2.4 Characterization of the reaction products by GC/MS and NMR
In the present work, we conducted a very careful analytical procedure, in order to ensure that we collected all the possible products and give both a reliable analysis and a complete carbon mass balance.Finally, GC/MS analysis was employed to identify any minor reaction by-products and their detailed quantification is listed in Tables S1 and S2.† GC/MS analysis was carried out on a Waters GCT premier system using an Agilent DB-5MS capillary column [30 m (L) × 0.25 mm (OD) × 0.25 μm (film)]. The oven temperature profile employed to achieve the required separation was 40 °C held for 5 min followed by a temperature ramp of 8° min−1 up to 280 °C, with this final temperature being held for 5 min. The injector temperature employed was 200 °C. Because the total conversion is calculated from the molar amount of cyclohexane which decreases during the reaction, the total selectivity is equal to the mass balance of all the observed products which means that our quoted conversion values may possibly be very slightly over-estimated.
Product screening was also performed using a Bruker Avance III 600 MHz NMR spectrometer equipped with a QCI cryo-probe. The extracted solid product in the MB-catalysed cyclohexane oxidation was also analysed by NMR. Both 1H-NMR spectra are presented in Fig. S1† and these show that we have not missed any higher molecular weight products that are not amenable to GC analysis. In the case of (i) MB catalysed cyclohexane oxidation and (ii) cobalt naphthenate catalysed cyclohexane oxidation, no additional products were detected.
3. Results and discussion
3.1 Characterization of the structure of MB using scanning transmission electron microscopy
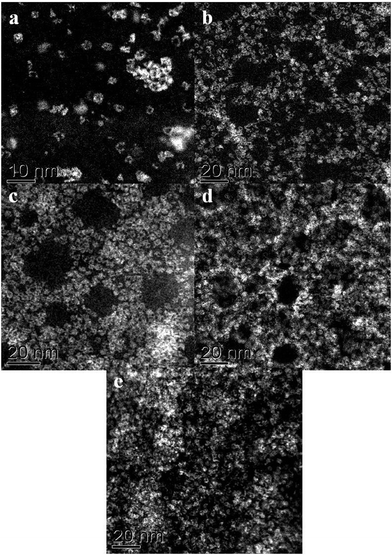
We have applied STEM-HAADF imaging for the first time to examine the nanostructure of MB samples. The first convincing structural models of MB, published by Müller et al. in 1995, were primarily based on powder X-ray diffraction and the evidence suggested that the material was comprised of complex assemblies of wheel-shaped mixed-valence polyoxomolybdate [MoVI126MoV28O462H14(H2O)70]14− anion clusters.5,6 In our work, samples for STEM characterization were prepared by putting a drop of a very dilute aqueous solution of MB onto a continuous carbon TEM grid and allowing the water to evaporate. Using this methodology any pre-existing self-assembled superstructures of rings are not retained at this high level of dilution and this method aids the study of the individual nano-rings rather than the superstructures. The MB ring structures are essentially invisible against the carbon support by conventional bright field phase contrast imaging (Fig. 1a), but are clearly visible in the HAADF image (Fig. 1b) by virtue of the strong atomic mass (z-) contrast generated by the Mo atoms in relation the other elements in the ring molecule and the C atoms in the underlying support film.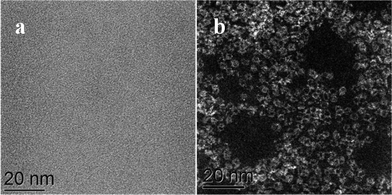 | ||
| Fig. 1 Comparison of the same area of an MB catalyst deposited on a continuous C film when imaged in (a) bright field (BF)-STEM mode and (b) high angle annular dark field (HAADF)-STEM mode. | ||
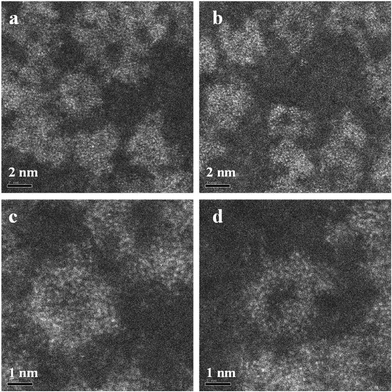
In very dilute samples the individual Mo154 wheel shaped clusters became well dispersed and can be imaged as separate entities (Fig. 2a), whereas in specimens generated from more concentrated MB solutions, the rings overlap giving a more confused picture due to their agglomeration into new superstructures as the solvent evaporates during the drop casting process (Fig. 2b–d).6 Low pass filtered HAADF images of individual MB units were obtained in which the ring structure can now be clearly observed (Fig. 3a). The rings are observed to have external and internal diameters of ~3.6 nm and ~2.0 nm respectively which matches well with the expected dimensions of the Mo154 model structure (inset of Fig. 3a). It should be noted that these ring structures are highly sensitive to prolonged electron irradiation damage at 200 kV and within a minute of exposure to the electron beam will collapse and transform into dense crystallites of MoO3 or even MoO2 as shown in sequence Fig. 3(a)–(e).24 This observation is in good agreement with previous HRTEM studies of reduced molybdenum oxides and molybdenum-blue-like species which have noted the propensity of these materials to exhibit electron beam modification.8 Aberration corrected STEM-HAADF imaging at 80 kV has also been performed on our MB materials (Fig. 4), and although the lower accelerating voltage was found to reduce the rate of electron beam damage somewhat, it did not eliminate the damage effects completely. We have also performed comparisons of the MB structure before and after the materials have been used as a catalyst for the oxidation of cyclohexane (Fig. 5: – the catalysis experiments are described in section 3.2). It was not possible to discern any significant difference between the pre- and post-use structures, confirming that the Mo154 ring morphologies are robust and remain intact under the mild reaction conditions employed (section 3.2).
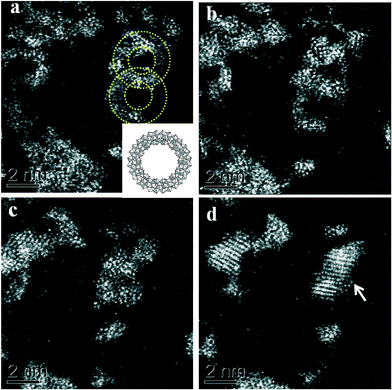 | ||
Fig. 3 (a–d) A set of HAADF-STEM micrographs (low-pass filtered) of a pair of nano-rings (circled in (a)) showing the effect of electron beam irradiation on the MB material at 200 kV. The images (a–c) were acquired sequentially, using a 17 s total exposure time (~50 pA, 200 kV, 512 × 512, 64 μs per pixel) for each micrograph. Image (d) was taken under the same conditions, but after 3 minutes total exposure time. A dense crystalline phase is seen to develop from the ring structures with increasing exposure time. The crossed fringe structure in (d) can be ascribed to the [1![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 4] projection of α-MoO3 (PDF# 04-008-4311) in which the (1 4] projection of α-MoO3 (PDF# 04-008-4311) in which the (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and (71 ) and (71![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) lattice planes are resolved with an intersection angle of ~83.4°. Inset in (a) is a schematic diagram of a single [MoVI126MoV28O462(H2O)70]14− ring-type molecule (after ref. 5). ) lattice planes are resolved with an intersection angle of ~83.4°. Inset in (a) is a schematic diagram of a single [MoVI126MoV28O462(H2O)70]14− ring-type molecule (after ref. 5). | ||
When more concentrated solutions of MB were drop-cast onto the carbon support films, we found evidence that the [MoVI126MoV28O462H14(H2O)70]14− anion clusters were prone to re-assemble under the influence of hydrogen bonding into periodic layered super-structures typically containing 20 nm-scale mesopores (see Fig. 5 and 6).5 The ring spacings and separation in Fig. 6(a) are broadly consistent with the structure (Fig. 6(b)) of the Na16[MoVI124MoV28O429(3-O)28H14(H2O)66.5]ca. 300H2O ({Mo152}) compound as determined previously by XRD, although the counter-ion present in our case is more likely to be H+.5,7,25 It should be noted that such self-assembled superstructures could not be directly imaged in a previous STM study of Zhong et al., where the individual Mo154 moieties had to be well separated on flat surface in order to acquire an image.6,9 The mixed MoVI/MoV valence state, coupled with a high effective surface area (240 m2 g−1) should in principle make this a highly attractive and effective catalyst morphology for the oxidation of cyclohexane.
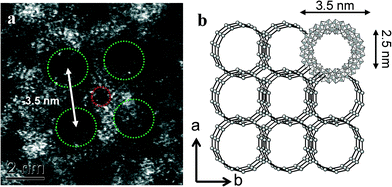 | ||
| Fig. 6 (a) A high magnification HAADF image of a MB sample which was prepared from a more concentrated MB solution, the Mo154 rings have assembled into a more ordered layered superstructure. The hole spacings and separation in (a) are broadly consistent with the structure (b) of the as self-assembled superstructure determined previously by XRD (after ref. 5 and 7). | ||
3.2 Cyclohexane oxidation using MB as a catalyst
We noted that there are abundant electron transitions between neighbouring MoVI/MoV cations in MB and that it has a redox potential that is very similar to FeIII/FeII in Keggin polyoxoanions.26,27 This suggests that MB could be a good candidate as a catalyst in some challenging selective oxidation reactions. For this reason we have explored whether or not the MoV–O–MoVI bridging structure in MB is active for selective redox chemistry6,8–13 by testing it for the oxy-functionalization of cyclohexane.In this work the activity of MB as a catalyst for cyclohexane (C6H12) oxidation has been contrasted with that of cobalt naphthenate which is currently used as a homogeneous catalyst in commercial processes. We also compare the activity of MB with that of Fe(acac)3, which is widely used as an iron resource for the aerobic oxidation of cyclohexane.14 For the experiments with MB, as we were investigating the use of very low amounts of the material as a catalyst, we first made a significantly dilute aqueous solution of MB and put a small drop of this with desired volume into the glass reaction vessel to give the required amount of MB. The solution was then evaporated and hence this pre-treatment is analogous to that used in the preparation of the STEM samples and we know that for dilute MB samples that this results in the formation of discrete nano-ring structures. Hence this permits us to study the catalytic performance of the individual nano-ring structures without the complication of the presence of the more complex self-assembled super-structures. In our initial experiments with MB we observed a marked catalytic activity of 6% conversion with 93% selectivity to oxygenated products for this (Table 1, Fig. 7, and ESI† Table S1, Fig. S1(a)–(b) and S2). At this level of cyclohexane conversion the performance is similar to that of the cobalt naphthenate commercial catalyst and ferric acetylacetonate (Table 1 and ESI† Table S2, Fig. S1(c)–(d)). We analysed the reaction mixtures in detail using a range of analytical techniques to ensure that all products were correctly identified (see section 2). The major products detected were cyclohexanol, cyclohexanone and adipic acid. Traces amounts of hexyl esters were also detected. Furthermore, we have ensured that the mass balance was closed and that any differences observed in selectivity between MB and cobalt naphthenate were reproducible and statistically significant.
| Catalyst | Conversiona (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| A | K | CHHP | AA | Totalb | |||
| a Reaction conditions, unless otherwise specified: cyclohexane (8.5 g), catalyst (6 mg), 140 °C, 3 bar O2, 17 h reaction time. In the shorthand notation used, Fe(acac)3 is Fe(III) acetylacetonate; A, K, CHHP, and AA represent cyclohexanol, cyclohexanone, cyclohexyl hydroperoxide and adipic acid respectively.b By-products include long chain carbons and esters (see ESI,† Tables S1 and S2).c MB sample after thermal treatment (see section 3.2). | |||||||
| 1 | MB | 6.0 | 52 | 40 | 0 | 1 | 93 |
| 2 | Co-naphthanate | 9.0 | 45 | 37 | 0 | 2 | 85 |
| 3 | Fe(acac)3 | 7.7 | 35 | 48 | 0 | 0 | 82 |
| 4 | SiMo12O40 | 6.0 | 30 | 50 | 0 | 9 | 90 |
| 5 | Blank | 1.1 | 22 | 19 | 57 | 0 | 98 |
| 6 | MBc | 5.5 | 28 | 49 | 0 | 20 | 98 |
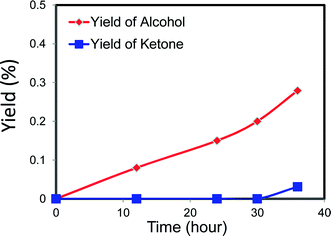 | ||
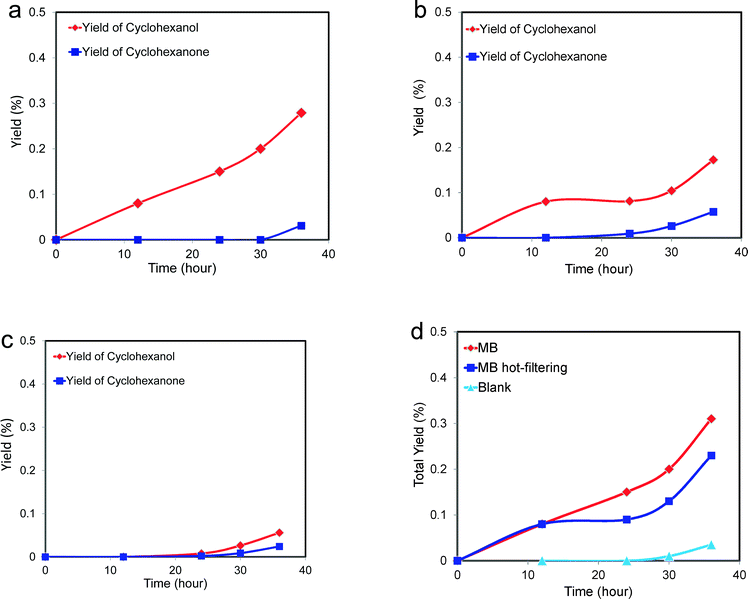
| Fig. 7 Catalytic performance of MB-catalysing cyclohexane oxidation as a function of reaction time (data for a hot-filtration experiment following this reaction is shown in Fig. 9). Reaction conditions 8.5 g cyclohexane, 6 mg MB, 120 °C, 3 bar O2, glass reactor. Alcohol, ketone, AA and CHHP represent cyclohexanol, cyclohexanone, adipic acid and cyclohexyl hydroperoxide, respectively. | ||
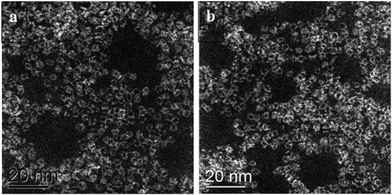
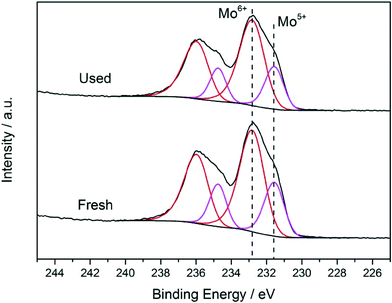
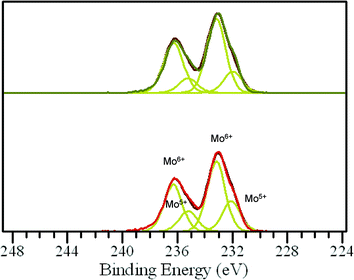
These catalytic data show that MB can be a promising material for the oxidation of this organic substrate, but it was also important to determine if catalysts used in batch reaction conditions can be effectively re-used. From the results shown in Table 2, it is apparent that the MB catalyst is fully re-usable based upon the measurements taken over four sequential usage cycles. Moreover, HAADF images of the MB before and after use in the oxidation of cyclohexane (Fig. 5a and b respectively) show that the characteristic Mo154 ring structure has been retained even after the material has been used as a catalyst. The oxidation state of the metal also did not change during the reaction. In fact, XPS experiments (Fig. 8) show that no remarkable differences were observed between the XPS profiles of the fresh and used MB material, with the ratio of MoV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MoVI cations remaining at about 1
MoVI cations remaining at about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, which is consistent with the previously reported molar ratio of 1
4, which is consistent with the previously reported molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as confirmed by the cerimetric titration method.28
4 as confirmed by the cerimetric titration method.28
| Number of usage cycles | Conversiona (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| A | K | CHHP | AA | Totalb | ||
| a Reaction conditions, cyclohexane (8.5 g), catalyst (6 mg), 140 °C, 3 bar O2, 17 h reaction time. In the shorthand notation used, A, K, CHHP and AA represent cyclohexanol, cyclohexanone, cyclohexyl hydroperoxide and adipic acid respectively. b By-products include long chain carbons and esters. | ||||||
| 1st | 6.0 | 52 | 40 | 0 | 1 | 93 |
| 2nd | 6.5 | 50 | 42 | 0 | 0 | 92 |
| 3rd | 6.3 | 53 | 42 | 0 | 2 | 97 |
| 4th | 6.3 | 52 | 42 | 0 | 1 | 95 |
| Catalyst | Conversiona (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| A | K | CHHP | BCH | AA | Total | ||
| a Reaction conditions: 8.5 g cyclohexane, catalyst (6 mg), 140 °C, 3 bar O2, 17 hour; Fe(acac)3 represents Fe(III) acetylacetonate. In the shorthand notation used, A, K, CHHP, AA and BCH represent cyclohexanol, cyclohexanone, cyclohexyl hydroperoxide, adipic acid and bromocyclohexane respectively. | |||||||
| MB | 7 | 58 | 26 | — | 9 | — | 93 |
| Co naphthenate | 0.9 | 3 | 12 | — | 75 | — | 90 |
| Fe(acac)3 | 0.8 | 5 | 7 | — | 35 | — | 47 |
| SiMo12O40 | 6.7 | 50 | 35 | — | 10 | — | 95 |
In the presence of CBrCl3, we observed that the activity of MB remains basically unaltered by the addition of the scavenger, whereas the activity of Co-naphthenate and Fe(acac)3 is severely suppressed, with their conversions reduced by about one order of magnitude (Table 3). Even more significant, in presence of the scavenger the selectivity for Co-naphthenate and Fe(acac)3 is now shifted towards cyclohexanone as the major product (ca. 10%), whereas, in case of MB the selectivity is shifted even more towards the more desirable cyclohexanol ( i.e. from 52% to 58%), which would suggest a real catalytic control exists in the MB system.
In order to identify the underlying source of this behaviour, we have systematically studied the reactivity of MB nanorings, especially considering the specific structure of MB described in section 3.1. The first comparison was a control reaction using molybdate silicate blue (SiMo12O40) which is stable in our reaction environment and also exhibits a mixed valence MoV–O–MoVI structure.33 The first observation is that this compound also shows similar conversion values of MB and also is not quenched by adding CBrCl3 (see Tables 1 and 3). Moreover, the SiMo12O40 is also capable of creating a higher selectivity towards the alcohol, unlike Co-naphthenate and Fe(acac)3, thus suggesting that the structural motif of mixed valence MoV–O–MoVI is important for the catalysis. Although it should be stressed that this effect for SiMo12O40 is fully evident in presence of radical scavenger only (Table 3). In fact, it is under these conditions that it is possible to discriminate between the autoxidation pathway and the activity induced by SiMo12O40.
On the other hand, the presence of oxygen in the MB lattice could also play a role in the process to explain this reactivity. In other words, if oxygen is removed from the lattice during the reaction, that in turn disrupts the MoV/MoVI moiety, which could alter the catalytic activity, possibly in terms of both conversion and selectivity. Therefore, to demonstrate that the oxygen from the MB structure is playing a role in the catalytic activity, we have carried out one further set of experiments. It is known that treating MoO3 at temperatures above 140 °C under anaerobic conditions can deplete some of the lattice oxygen and thus affect its reactivity.34 These effects have been investigated for a temperature of 180 °C and this did not disrupt the three-dimensional lattice framework of the metal oxide.35 Consequently, we thermally treated some MB at 180 °C for 48 h under a flow of He with the aim to achieve the same results observed for MoO3, i.e. alteration of the MoV/MoVI moiety without collapse of the three dimensional MB framework.36 The material was then examined by means of XRD and STEM in order to determine if the ring structures and molecular crystal framework superstructure were left intact after heating. In addition, it was further characterized by means of XPS and DR-UV to assess if the MoV/MoVI motif was disrupted or not, and tested again as a catalyst afterwards.
A comparison of the XRD patterns before and after thermal treatment shows that these two patterns are nearly identical (ESI,† Fig. S3 and S4). This suggests that the overall MB structure (both the nanoring and super-structure) remained intact, and if any changes took place, this was related to O removal only, which can be accommodated without any large scale collapse of the structure. It should be stressed that the powder X-ray patterns that we collected for our MB samples (ESI† Fig. S3) are in full agreement with the XRD characterization carried out by Müller et al. on dry powders of MB,6i.e. diffuse scattering maxima at ca. 26°, 29.5°, 2θ and a broad set at 51° 2θ degrees. In addition, by STEM we still see rings of similar dimension after the heat treatment in He (ESI† Fig. S5(a)–(d)). However, with respect to the ratio of MoV/MoVI units present we do see a measurable difference. In the fresh sample, by XPS analysis (Fig. 10) we observe that the MoV molar fraction is 20%, whereas after thermal treatment in He, it increases to 30%. Hence a clear increase in MoV and corresponding decrease in MoVI species is observed in the thermally-treated MB sample, the other XPS parameters, such as peak position and number of species present, remain unchanged, implying that the MB ring structure remains intact, and that the change in MoV/MoVI ratio is due to some removal of oxygen from the backbone of the ring lattice during the thermal treatment in He.
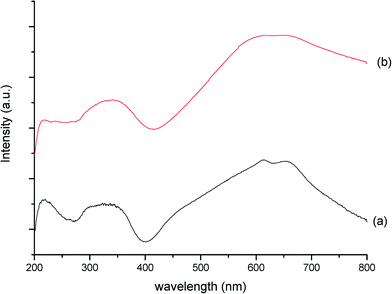
Additional evidence from DR-UV/vis spectra (Fig. 11) provides a further important indication to what has happened to the MB material during this detrimental thermal treatment. The inter-valence charge transfer (IVCT) bands at 600–700 nm,37 a characteristic finger-print region for molybdenum-blue compounds,38 have merged into one band, confirming that a change in electronic properties has occurred. As these inter-valence bands are due to MoV–O–MoVI entities, their modification can be attributed to either loss of lattice oxygen, or breaking of some of the MoV–O–MoVI linkages.
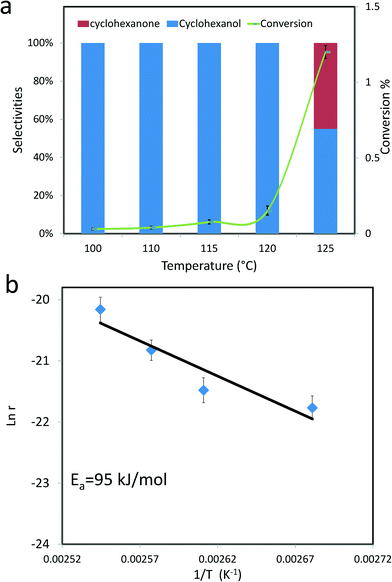
All this data suggests that the thermal treatment in He left the ring structure intact, but disrupted the MoV–O–MoVI centres thus providing us with the opportunity to further assess the role of these MoV–O–MoVI moieties on the catalytic activity. Catalytic testing for the MB thermally treated in He showed that the conversion was basically unmodified (ca. 6%), whereas the selectivity to the alcohol was markedly decreased from 52% to 22%, (compare entries 1 and 6 in Table 1) and the selectivity is now shifted towards cyclohexanone and adipic acid (at ~49% and 20% respectively) the latter of which was initially absent. This selectivity change is a key experimental observation, because if MB were functioning solely as an initiator, i.e. a promoter of autoxidation pathways like Fe(acac)3 or Co-naphthenate, then the selectivities would remain the same, which is not observed. This experiment also serves to show that the number of MoV–O–MoVI moieties present is a key factor for determining the amount of alcohol formation. In fact, for Co-naphthenate or Fe(acac)3 the reaction has been established to proceed by means of a Haber–Weiss cycle39,40 which involves homolytic cleavage of the cyclohexyl hydroperoxide intermediate. In contrast, for MB, the reaction could conceivably take place via an oxo-metal species and thus proceed via a different mechanism to the autoxidation pathway.18,22 On this particular aspect, we should note that in this work solvent-free conditions have been employed and as such we have not explored the kinetics of the reaction when using diluted cyclohexane. The use of a solvent has not been attempted in this study, as it is known that solvents can have a strong unintended effect on the final product distribution,41,42 and we wanted to avoid further collateral complexity and undesired autoxidation reactions (Fig. 10 and 11). However, we have studied the catalytic activity of MB as a function of reaction temperature (Fig. 12a), and this shows a fascinating mono-hydroxylation of cyclohexane when using MB as a catalyst, which is clearly different from the autoxidation pathway which gives both alcohol and ketone as primary products.21 Hence we consider that this also indicates that MB is a real catalyst in the partial oxidation of cyclohexane. The activation energy of this process in the presence of MB is determined to be ~95 kJ mol−1 (Fig. 12b). Moreover, MB does not seem to be affected by oxygen diffusion limitations observed for some metal oxide based catalysts43 under our reaction conditions. And this could further extend its applicability.
4.0 Conclusions
Our results show that MB, a material that has largely been neglected as a catalyst to date, is a selective catalyst for C–H bond oxo-functionalisation, which has direct relevance for a number of important industrial processes. In addition we have provided the first scanning transmission electron microscopy images of MB which show that the structure stays intact during use and that the catalysis we observe is associated with the structural motif of mixed valence MoV–O–MoVI entities which are present in MB nanorings and related structures. Furthermore, a remarkable difference between the catalytic behaviour of MB and commercial Co-naphthenate and Fe(acac)3 catalysts was observed that allows some degree of product selectivity control when using MB. We consider that these observations will spur the design of a new generation of MB based catalysts with enhanced performance for alkane activation.Acknowledgements
The authors wish to acknowledge the support of INVISTA Textiles (UK) Limited, INVISTA Intermediates and INVISTA Technologies S. à r. l. In addition, M. W. and C. J. K. gratefully acknowledge funding from the National Science Foundation Major Research Instrumentation program (GR# MRI/DMR-1040229).Notes and references
- M. G. Mellon, Analytical Absorption Spectroscopy, John Wiley and Sons Inc., 1950 Search PubMed.
- P. M. Huang, Y. Li and M. E. Sumner, Handbook of Soil Sciences: Resource Management and Environmental Impacts, CRC Press, 2nd edn, 2011 Search PubMed, CH13-11.
- M. Pansu and J. Gautheyrou, Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods, Springer, 2007, p. 827 Search PubMed.
- C. W. Scheele and D. Sigismund Friedrich Hermbstädt, Sämmtliche physische und chemische Werke, 1793, pp. 185–200. Digital copy available at http://reader.digitale-sammlungen.de/de/fs1/object/display/bsb10915844_00001.html Search PubMed.
- A. Müller, J. Meyer, E. Krickemeyer and E. Diemann, Angew. Chem., Int. Ed. Engl., 1996, 35, 1206 CrossRef.
- D. Zhong, F. L. Sousa, A. Müller, L. Chi and H. Fuchs, Angew. Chem., Int. Ed., 2011, 50, 7018 CrossRef CAS PubMed.
- A. Müller and C. Serain, Acc. Chem. Res., 2000, 33, 2 CrossRef PubMed.
- P. Delporte, F. Meunier, C. Pham-Huu, P. Vennegues, M. J. Ledoux and J. Guille, Catal. Today, 1995, 23, 251 CrossRef CAS.
- T. Liu, E. Diemann, H. Li, A. W. Dress and A. Müller, Nature, 2003, 426, 59 CrossRef CAS PubMed.
- D. M. David Jeba Singh, T. Pradeep, J. Bhattacharjee and U. V. Waghmareb, J. Am. Soc. Mass Spectrom., 2007, 18, 2191 CrossRef PubMed.
- B. Visic, M. Klanjsek Gunde, J. Kovac, I. Iskra, J. Jelenc and M. Remskar, Mater. Res. Bull., 2013, 48, 802 CrossRef CAS PubMed.
- G. Li, L. Jiang, S. Pang, H. Peng and Z. Zhang, J. Phys. Chem. B, 2006, 110, 24472 CrossRef CAS PubMed.
- Y. Kikutani, J. Mol. Catal. A: Chem., 1999, 142, 247 CrossRef CAS.
- S. A. Schunk, Handbook of Heterogeneous Catalysis, Wiley-VCH, Weimar, 2nd edn, 2008 Search PubMed.
- A. M. Khenkin, L. Weiner, Y. Wang and R. Neumann, J. Am. Chem. Soc., 2001, 123, 8531 CrossRef CAS PubMed.
- R. K. Rana and B. Viswanathan, Catal. Lett., 1998, 52, 25 CrossRef CAS.
- R. S. da Cruz, M. M. Dauch, U. Schuchardt and R. Kumar, Stud. Surf. Sci. Catal., 2000, 130, 1037 CrossRef.
- J. A. Labinger, J. Mol. Catal. A: Chem., 2004, 220, 27 CrossRef CAS PubMed.
- R. H. Crabtree, J. Chem. Soc., Dalton Trans., 2001, 2437 RSC.
- U. Schuchardt, D. Cardoso, R. Sercheli, R. Pereira, R. S. da Cruz, M. C. Guerreiro, D. Mandelli, E. V. Spinace and E. L. Pires, Appl. Catal., A, 2001, 211, 1 CrossRef CAS.
- I. Hermans, P. A. Jacobs and J. Peeters, Chem. – Eur. J., 2006, 12, 4229 CrossRef CAS PubMed.
- B. P. C. Hereijgers and B. M. Weckhuysen, J. Catal., 2010, 270, 16 CrossRef CAS PubMed.
- S. B. Turnipseed, A. J. Allentoff and J. A. Thompson, Anal. Biochem., 1993, 213, 218 CrossRef CAS PubMed.
- D. Wang, D. S. Su and R. Schlögl, Z. Anorg. Allg. Chem., 2004, 630, 1007 CrossRef CAS.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann, C. Beugholt, S. K. Das and F. Peters, Chem. – Eur. J., 1999, 5, 1496 CrossRef.
- S. Noro, R. Tsunashima, Y. Kamiya, K. Uemura, H. Kita, L. Cronin, T. Akutagawa and T. Nakamura, Angew. Chem., Int. Ed., 2009, 48, 8703 CrossRef CAS PubMed.
- J. M. Poblet, X. López and C. Bo, Chem. Soc. Rev., 2003, 32, 297 RSC.
- C. M. Callahan, S. C. Foti and J. R. Lai, Anal. Chem., 1960, 32, 635 CrossRef CAS.
- M. Conte, X. Liu, D. M. Murphy, K. Whiston and G. J. Hutchings, Phys. Chem. Chem. Phys., 2012, 14, 16279 RSC.
- S. V. Dvinskikh, A. V. Yurkovskaya and H.-M. Vieth, J. Phys. Chem., 1996, 100, 8125 CrossRef CAS.
- C. Berti and M. J. Perkins, J. Chem. Soc., Chem. Commun., 1979, 1167 RSC.
- A. Dhakshinamoorthy, M. Alvaro and Hermenegildo García, ACS Catal., 2011, 1, 48 CrossRef CAS.
- Y. Kikutani, J. Mol. Catal. A: Chem., 1999, 142, 247 CrossRef CAS.
- M. Labanowska, Phys. Chem. Chem. Phys., 1999, 1, 5385 RSC.
- M. Conte and V. Chechik, Chem. Commun., 2010, 46, 3991 RSC.
- T. Ressler, J. Wienold, R. E. Jentoft and F. Girgsdies, Eur. J. Inorg. Chem., 2003, 301 CrossRef CAS.
- A. A. Bugayev and S. E. Nikitin, Opt. Commun., 2000, 180, 69 CrossRef CAS.
- B. P. C. Hereijgers and B. M. Weckhuysen, J. Catal., 2010, 270, 16 CrossRef CAS PubMed.
- S. Tanase, E. Bouwman and J. Reedijk, Appl. Catal., A, 2004, 259, 101 CrossRef CAS PubMed.
- R. H. Holm, Chem. Rev., 1987, 87, 1401 CrossRef CAS.
- J. Bäckvall, Modern Oxidation Methods, Wiley, 2nd edn, 2011 Search PubMed.
- I. Rekkab-Hammoumraoui, A. Choukchou-Braham, L. Pirault-Roy and C. Kappenstein, Bull. Mater. Sci., 2011, 34, 1127 CrossRef PubMed.
- C. Hettige, K. R. R. Mahanama and D. P. Dissanayake, Chemosphere, 2001, 43, 1079 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of GC/MS and NMR characterization of the products and XRD and STEM of MB after thermal treatment. See DOI: 10.1039/c4cy01213e |
| This journal is © The Royal Society of Chemistry 2015 |