Open Access Article
Open Access ArticleSemimetal-functionalised polyoxovanadates
Kirill Yu.
Monakhov
*a,
Wolfgang
Bensch
*b and
Paul
Kögerler
 *ac
*ac
aInstitut für Anorganische Chemie, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany. E-mail: kirill.monakhov@ac.rwth-aachen.de; paul.koegerler@ac.rwth-aachen.de
bInstitut für Anorganische Chemie, Christian-Albrechts-Universität zu Kiel, Max-Eyth-Str. 2, 24118 Kiel, Germany. E-mail: wbensch@ac.uni-kiel.de
cJülich-Aachen Research Alliance (JARA-FIT) and Peter Grünberg Institute (PGI-6), Forschungszentrum Jülich, 52425 Jülich, Germany
First published on 7th September 2015
Abstract
Polyoxovanadates (POVs), known for their wide applicability and relevance in chemical, physical and biological sciences, are a subclass of polyoxometalates and usually self-assemble in aqueous-phase, pH-controlled condensation reactions. Archetypical POVs such as the robust [VIV18O42]12− polyoxoanion can be structurally, electronically and magnetically altered by heavier group 14 and 15 elements to afford Si-, Ge-, As- or Sb-decorated POV structures (heteroPOVs). These main-group semimetals introduce specific chemically engineered functionalities which cause the generally hydrophilic heteroPOV compounds to exhibit interesting reactivity towards organic molecules, late transition metal and lanthanoid ions. The fully-oxidised (VV), mixed-valent (VV/VIV and VIV/VIII), “fully-reduced” (VIV) and “highly-reduced” (VIII) heteroPOVs possess a number of intriguing properties, ranging from catalytic to molecular magnet characteristics. Herein, we review key developments in the synthetic and structural chemistry as well as the reactivity of POVs functionalised with Si-, Ge-, As- or Sb-based heterogroups.
Kirill Monakhov received his Dr rer. nat. degree in 2010 with Prof. Gerald Linti (Heidelberg University, Germany). After two years as a postdoctoral fellow of the German Research Foundation and the Cercle Gutenberg with Prof. Pierre Braunstein (University of Strasbourg, France), and after being awarded the Academia Europaea Burgen Scholarship in 2011, he returned to Germany in 2013 with a DFG postdoctoral reintegration fellowship to join the group of Paul Kögerler at RWTH Aachen University. In 2015 he received a DFG Emmy Noether fellowship and now leads a junior research group at the Institute of Inorganic Chemistry at RWTH Aachen. |
Wolfgang Bensch received his Dr rer. nat. with Prof. Eberhard Amberger at the Ludwig-Maximilians-University of Munich (Germany) in 1983. He was a postdoctoral fellow at the University of Zurich (Switzerland) and joined Siemens Company (Munich) in 1986. In 1990 he started his habilitation at the Johann Wolfgang Goethe University Frankfurt (Germany) which was finished in 1993. He was appointed as full professor for Inorganic Solid State Chemistry at the Christian-Albrechts-University Kiel in 1997. |
Paul Kögerler graduated with a Dr rer. nat. degree with Prof. Achim Müller at the University of Bielefeld (Germany) in 2000, followed by a postdoctoral research stay at the Department of Physics and Astronomy at Iowa State University (USA). In 2003, he was appointed as a tenured Associate Scientist at the U.S. DOE Ames Laboratory, before returning to Germany in 2006 as Professor of Chemistry at the Institute of Inorganic Chemistry at RWTH Aachen University and Group Leader for Molecular Magnetism at the Peter Grünberg Institute, Research Centre Jülich. |
1. Introduction
1.1 General remarks
Although vanadium is best known for its use in the large-scale industrial production of alloys and steels, this group 5 transition metal also plays a significant role in biological systems and bioinorganic chemistry.1,2 Vanadium shows common oxidation states between +2 and +5 revealed by characteristic colours such as lilac/purple (V2+), green (V3+), blue ({VIVO}2+), and yellow ({VVO2}+ and {VVO3}−) and exhibits a particularly rich coordination chemistry in aqueous solutions.3,4 It has a high tendency to form oxovanadium ions whose nuclearity, structural motifs and net charge are strongly influenced by the specific reaction conditions such as stoichiometries and concentration of the reactants, pH (alkaline vs. acidic aqueous solutions), temperature, pressure, and reaction time. In general, vanadium oxide compounds find a wide range of applications in catalysis,5 biochemistry,1,2,6 sol–gel chemistry,7 gas sensing,8 geochemistry,9 sorption10 and intercalated layered material,11 surface and nano sciences12 and perform their role as secondary electrode materials for advanced lithium ion batteries and vanadium redox-flow batteries.13 Even in photosynthesis the role of vanadate and vanadyl citrate compounds was investigated.14 The chemistry of polyoxovanadates (POVs),15 the latter being a subclass of polynuclear molecular early transition metal oxides known as polyoxometalates (POMs),16 is a very fast growing area of research, mainly due to the versatile redox activities of POVs,17 their current application and further perspectives in various branches of chemical, physical and biological sciences.1.2 Structure and characterisation of conventional POVs
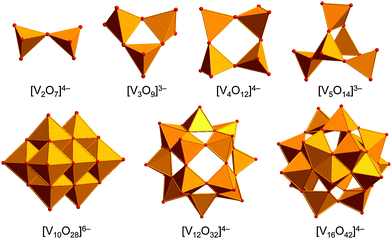
In aqueous and organic18 solutions, POVs are formed in pH-dependent condensation reactions in which small [VOn]Q− fragments aggregate to form a large variety of high- and low-nuclearity cluster structures with diverse coordination geometries of the vanadium cations. POVs usually exhibit cage-, sphere-, hollow-, basket-, belt-, and barrel-like structural motifs,19 which are constructed of a number of polyhedra fused and/or linked through a common vertex (corner) and/or polyhedral edges and faces. These polyhedra consist of the homo- or heterovalent V atoms showing e.g. square-pyramidal20 [VVO5]5− and [VIVO5]6−, octahedral [VVO6]7− and [VIVO6]8− and tetrahedral [VVO4]3− coordination geometries. Thus, the adjustable coordination behaviour of V in POVs contrasts with the predominantly octahedral coordination environments of Mo and W ions in their POM structures. One of the most studied and structurally characterised POVs is the orange-coloured decavanadate ion,21 [V10O28]6− (Fig. 1), that demonstrates the ability to build supramolecular assemblies22 and shows fascinating biological activity.23 This polyanion, reported for the first time in 1956, has also been found in minerals (see, e.g. lasalite, Na2Mg2[V10O28]·20H2O).24POVs can be divided into four general families: fully-oxidised (VV), mixed-valent (VV/VIV or VIV/VIII), “fully-reduced” (VIV) and “highly-reduced” (VIII) species. The following crystallographically characterised POVs [V2O7]4−,25 [V3O9]3−,26 [V4O12]4−,27 [V5O14]3−,28 [V10O28]6−,29 [V12O32]4−,30 [V13O34]3−,31 [V15O42]9−,32 [V16O42]4−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 constitute the class of fully-oxidised vanadium species (Fig. 1). X-ray single-crystal structures have been determined for [VIV2VV8O26]4−,34 [VIV8VV7O36]5−,35 [VIV11VV5O38]7−,36 [VIV3VV13O42]7−,37 [VIV5VV12O42]4−,38 [VIV16VV2O42]10−,39 [VIV10VV8O42]4−,39 [VIV8VV10O44]6−,40 [VIV6VV13O49]9−,41 [VIV8VV14O54]6−,40 [VIV16VV18O82]10−
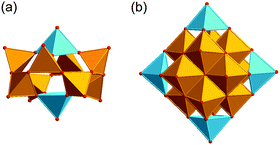
33 constitute the class of fully-oxidised vanadium species (Fig. 1). X-ray single-crystal structures have been determined for [VIV2VV8O26]4−,34 [VIV8VV7O36]5−,35 [VIV11VV5O38]7−,36 [VIV3VV13O42]7−,37 [VIV5VV12O42]4−,38 [VIV16VV2O42]10−,39 [VIV10VV8O42]4−,39 [VIV8VV10O44]6−,40 [VIV6VV13O49]9−,41 [VIV8VV14O54]6−,40 [VIV16VV18O82]10−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 (disregarding encapsulated supramolecular guest species) from the class of the mixed-valent VV/VIV species (Fig. 2). The mixed-valent VIV/VIII-POVs and the “highly-reduced” VIII-POVs consist mostly of a variety of vanadium alkoxide structures.43,44 The most renowned representative in the class of the “fully-reduced” POVs is the [VIV18O42]12− polyoxoanion whose chemical and structural characterisation were reported for the first time by Johnson and Schlemper in 1978 (Fig. 3).45 The {V18O42} architecture constructed from the edge-sharing square-pyramidal {O
42 (disregarding encapsulated supramolecular guest species) from the class of the mixed-valent VV/VIV species (Fig. 2). The mixed-valent VIV/VIII-POVs and the “highly-reduced” VIII-POVs consist mostly of a variety of vanadium alkoxide structures.43,44 The most renowned representative in the class of the “fully-reduced” POVs is the [VIV18O42]12− polyoxoanion whose chemical and structural characterisation were reported for the first time by Johnson and Schlemper in 1978 (Fig. 3).45 The {V18O42} architecture constructed from the edge-sharing square-pyramidal {O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) VO4} units represents an archetypal structure with a diameter of ca. 11 Å which may adopt idealised Td and D4d symmetries. The strong infrared stretching frequencies of terminal V
VO4} units represents an archetypal structure with a diameter of ca. 11 Å which may adopt idealised Td and D4d symmetries. The strong infrared stretching frequencies of terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups characteristically appear in the range 940–1000 cm−1.
O groups characteristically appear in the range 940–1000 cm−1.
The presence of heterovalent vanadium atoms in some POVs occasionally complicates the assignment of the positions of the individual V atoms. This is due to the multifaceted nature of the POV structures, which often afford similar coordination geometries (e.g. square-pyramidal) around the VV/VIV ions and feature charges which are delocalised over the vanadium centres. Generally, there are several approaches to assign the oxidation states of V atoms in the entire POV structure: (i) overall charge balance, (ii) calculation of bond valence sums from determined bond lengths,46 (iii) redox titration and (iv) X-ray photoelectron spectroscopy (XPS). Elemental, thermal (TG-DTA),47 X-ray powder diffraction and electron microprobe (EMP) analyses, infrared (IR) spectroscopy, electron paramagnetic resonance spectroscopy (EPR), 1H, 17O and 51V nuclear magnetic resonance spectroscopy (NMR),48 energy-dispersive X-ray spectroscopy (EDXS), ultraviolet-visible (UV-vis) spectroscopy, electrospray ionisation mass spectrometry (ESI-MS),49 cyclic voltammetry (CV), and inelastic neutron scattering (INS) have been used for the chemical and structural characterisation of POVs. Temperature- and field-dependent magnetic susceptibility measurements were routinely applied to evaluate the magnetic characteristics of reduced POVs. A study combining density functional theory (DFT) calculations and 51V NMR experiments for [V10O28]6− was published some time ago.50 More recently, joint experimental and computational studies have been performed on positively-charged vanadium oxide clusters in the gas phase and in solution. These involved e.g. the combination of laser desorption time-of-flight mass spectrometry, UV-vis and IR spectroscopy, and DFT calculations,51 or analysis by ESI-MS, collision-induced dissociation experiments and DFT.52
1.3 Relation of POVs to classical POMs
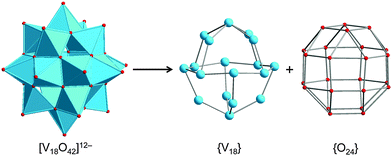
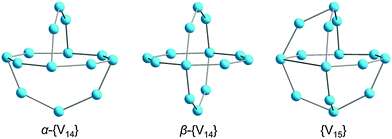
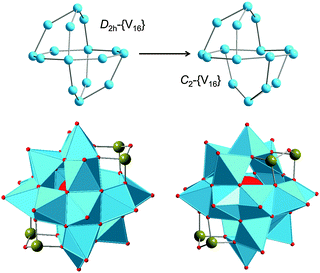
The POV structures are usually derived or deduced from transferable vanadium oxoanion building blocks and host–guest aggregates, but also from small stable POM archetypes. Although the redox behaviour of the POVs differs from that of POMs comprised of group 6 transition metals (M = Mo, W), some of the construction principles of the metal-oxide core frameworks are very similar between these two classes of compounds. The POVs have thus been shown to adopt the structures of the classical Lindqvist-type [M6O19]Q− polyanions,53 as exemplified by the mixed-valent [V6O19]Q− polyoxoanions,54 and to exhibit expanded Keggin-type [YM12O40]Q− structures (Y: heteroatom).55 A first representative in the latter family, a fully-oxidised [PV14O42]9− POV, which is composed of an α-Keggin [PV12O40]15− polyoxoanion capped by two VO3+ moieties, was reported in 1980.56 A mixed-valent bicapped polyoxomolybdate Keggin-type polyoxoanion with two {VO}2+ caps,57 [PMoV6MoVI6O40(VIVO)2]5−, is of interest due to its isostructural relations with the fully-oxidised [V15O42]9− isopolyoxovanadate. So-called superkeggin structures based on the {V18O42} constituents have been found for the mixed-valent complexes [VIV12VV6O42(SO4)]8−,58 [VIV18O42H9(VVO4)]6−,58 and [VIV14VV4O42(PO4)]11−.59 Note that the structure of the archetypal “fully-reduced” [VIV18O42]12− polyoxoanion with D4d symmetry can formally be derived stepwise from the fully-oxidised α-Keggin-type framework [VV12O36]12−, as illustrated in Fig. 4.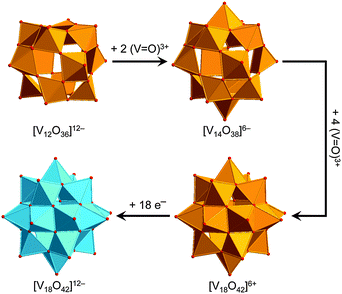 | ||
| Fig. 4 Schematic structural evolution of the fully-oxidised α-Keggin-type framework [V12O36]12− towards the “fully-reduced” [V18O42]12− POV via formation of the bicapped Keggin structure [V14O38]6− and the hypothetical [VV18O42]6+ structure, the latter here assumed as isostructural to [VIV18O42]12−. Colour code as in Fig. 2 and 3. | ||
1.4 ‘Host–guest’ complexation in POV chemistry
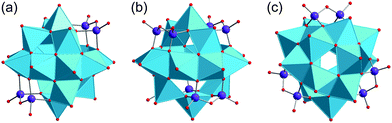
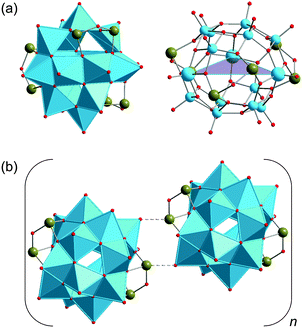
The cage-, basket-, barrel- and sphere-like shape of high-nuclearity POVs enables them to entrap small guest species60 in their central voids, in a manner that is structurally reminiscent of the “molecular container” behaviour of e.g. fullerenes. The case of a discrete (small) number of water molecules enclosed in a molecular container generally is of great interest: Jung and coworkers e.g. emphasise that “a single water molecule within an isolated space is a hot research topic in molecular science, owing to potential applications to delicate functions such as proton transfer, tautomerism, recognition, and biological systems”.61,62Fig. 5 illustrates that not only Pd(II) cage-type coordination complexes61 and fullerene C60,63 but also the hydrophilic POVs are able to host an individual H2O molecule.64 In the following, for reasons of clarity we indicate the supramolecular encapsulation state of a guest species by the “@” notation when specifying the composition of a discrete polyanion, whereas in full formulas for POV-based compounds the guest species is simply set in brackets.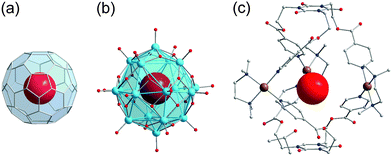 | ||
| Fig. 5 The H2O-endohedral (@) complexes of C60 (a),65 the “fully-reduced” [V18O42]12− POV (b),45,64 and [Pd3L2]6+ (c).61 C, grey; N, blue; O, red; VIV, sky blue; Pd, brown. | ||
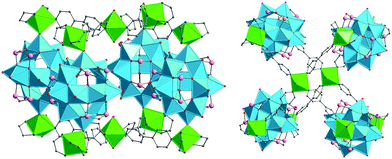
The prototypical fully-oxidised, mixed-valent and “fully-reduced” POV shells (e.g., bowl-like {V12O32}, nearly spherical D4d {V18O42}, ellipsoidal D2h {V18O44}, bulb-shaped D2d {V22O54}) were found to enclose not only water molecules but also anions (e.g., Cl−, Br−, I−, HCOO−, MeCOO−, CN−, CO32−, NO−, NO2−, N3−, PO43−, SH−, SCN−, SO32−, SO42−, ClO4−, and VO43−) within the vanadium oxide shells.39,66 Larger neutral guests (methanol, ethanol, ethane-1,2-diol) have furthermore been reported to exist in phosphate- and diaminopropane-capped {VIII3VIV18}-type cluster shells.67 In all of these compounds, the encapsulated halide, pseudohalide, inorganic or organic guest anion interacts with the anionic host shell solely via weak electrostatic and van der Waals forces at distances of ca. 3.5–4.0 Å. In this regard, the POV host structures68 can also be regarded as vanadium oxide matrices that isolate unstable guests with short lifetimes or high reactivities. The formation of POV host shells can also be controlled by templating effects of small guest anions present in solution.40,69 Interestingly, also certain cationic groups such as dimethylammonium that weakly bind to polyoxovanadate shell structures can assume the role of an interchangeable template as well, for example in the POV [Cl@VV12O32(H2NMe2)2]3− where one or both dimethylammonium groups can subsequently be exchanged by first-row transition metal cations.70 Macrocyclic, highly-oxidised POV structures [PdVV6O18]4−, [Cu2VV8O24]4−, and [Ni4VV10O30(OH)2(H2O)6]4− incorporating transition-metal cationic templates (Pd2+, Cu2+, and Ni2+) are also known.15,71
The far-reaching consequences for the properties of the host matrix are exemplified by a family of purely inorganic host–guest assemblies [X@HVIV8VV14O54]6− (X = VO2F2−, SCN−, and ClO4−).72 Here the nature of the guest (X) anion defined the magnetic and electrochemical characteristics of the virtually isostructural, mixed-valent VV/VIV-POV host matrices. Similarly, template-dependent reactivity has also been observed for the photooxidation of an organic dye by nearly isostructural [X@(Bi(dmso)3)2VV12O33]−-type POVs (X = Cl−, Br−).73 In general, POVs exhibiting host–guest relationships consist of transferable substructures and exist in a variety of reduction (and protonation) states. In the words of Müller et al.: “The template syntheses of new cluster compounds utilizing intrinsic host–guest relationships open up new possibilities for chemists to create nanosized host or cluster structures with novel magnetic properties”.74
1.5 Magnetism of POVs
The POVs can be magnetically functionalised either via their reduction towards the mixed-valent, “fully-reduced”, and “highly-reduced” structures comprising vanadyl {VIVO}2+ (isotropic spin-1/2) and {VIIIO}+ (spin-1) groups or via incorporation of paramagnetic transition metal or lanthanoid cations. The last few decades have shown that such POVs are ideal objects for magnetochemical studies75 and the exploration of nanoscale molecular magnetism76 due to their intriguing magnetic features, ranging from geometrical spin frustration to single-molecule magnet characteristics.77 Most commonly, the mixed-valent (VV/VIV or VIV/VIII), “fully-reduced” (d1-VIV) and “highly-reduced” (d2-VIII) vanadium spin-centres are coupled antiferromagnetically via bridging oxygen atoms, although ferromagnetic coupling between the vanadium ions is also possible, as has been forecasted in a quantum-chemical study of the synthetically readily accessible mixed-valent alkoxo-hexavanadates of the [VIV4VV2O7(OR)12] type.78 A comparative theoretical study of the magnetic properties of a homovalent [VIV18O42]12− POV with 18 fully localised valence electrons and its structurally identical mixed-valent derivative [VIV10VV8O42]4− with 10 partially delocalised electrons explored the role of electron transfer processes and magnetic exchange interactions in these structures.79 The preparation of the first water-soluble salt-inclusion solid [Cs11Na3(V15O36)Cl6] containing the mixed-valent [VIV11VV4O36Cl]9− building block is an interesting result in view of the development of “quantum magnetic solids within extended systems”.80,81 Notably, the chemical reactivity and acid-dissociation constants of fully and partly reduced POVs can in principle be influenced by the extent of charge delocalisation.1.6 Catalysis with POVs
The catalytic activity of the POV-based compounds was demonstrated by a number of authors. Gao and Hua reported that K7[NiIVV13O38]·16H2O containing the fully-oxidised [VV13O38]11− building block catalyses oxidative mineralisation of p-nitrophenol and p-chlorophenol into CO2, NO3−, and Cl− using 30% aqueous H2O2 as oxidant under mild conditions.82,83 Khan and coworkers investigated the selective catalytic reduction of nitrogen oxides into N2 by propylene as the reductant using the mixed-valent heterometallic compounds Li6[M3(H2O)12V18O42(YO4)]·24H2O (M = MnII, NiII; Y = V, S) and [M3(H2O)12V18O42(YO4)]·24H2O (M = FeII, CoII; Y = V, S) as precursors.84 The catalytic oxidative dehydrogenation of propane by the nanostructured compounds [M3(H2O)12V18O42(YO4)]·24H2O (M = Fe, Co, Mn; Y = V, S) has also been studied.85 Wu et al. showed that the compound (NH4)2[{Mn(salen)(H2O)}6V6O18](NO3)2·30H2O (salen2− = N,N′-(ethylene)bis(salicylideneiminate)), the structure of which comprises a cyclic POV anion decorated with six MnIII–salen Schiff-base complex groups, acts as photocatalyst for organic dye degradation.86 Chakrabarty and Banerjee studied the decomposition of H2O2 into O2 and H2O, which is catalysed by the [MnIVVV13O38]7− polyoxoanion in aqueous acetate buffer.87 The compound (NH4){[Zn4(dach)7(H2O)3][V3V18P6O60(dach)3]}·19H2O (dach = (±)-trans-1,2-cyclohexanediamine = C6H14N2) containing the nanoscale, highly-reduced [VIII3VIV18P6O60(dach)3]9− building block was shown to selectively catalyse styrene oxidation in the presence of H2O2.44 (NnBu4)4[V16O38(Br)] containing the mixed-valent [Br@VIV7VV9O38]4− building block exhibited catalytic activity in the oxidative bromination reactions of aromatic substrates under aerobic conditions.88 The mixed-valent (NnBu4)[VIV5VV1O7(OMe)12] compound was demonstrated to catalyse photoinduced water oxidation.891.7 Scope of the review
Embedding heteroelements into POV structures90 allows us to alter the electronic and magnetic properties, as well as the electrical conductivities91 of the resultant heteropolyoxovanadate (heteroPOV) spin structures (reminiscent of “doping” semiconducting materials) through the interplay between stoichiometries, heavy atom effects and redox potentials as well as steric and electronic environment around the terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Herein, we place emphasis on the structural variety of silicato-, germanato-, arsenato-, and antimonato-derivatised polyoxovanadates and their hybrid compounds with middle and late transition metals and discuss interesting molecular features and application perspectives of these heteroPOVs. Great interest in the polynuclear molecular vanadium oxides decorated with the heavier group 14 and 15 elements (E) is generated by the specific synthetic, structural, host–guest and vast redox chemistry and extraordinary magnetic characteristics of these compounds, involving e.g. geometrical spin-frustration.92 Remarkably, the structurally, electronically and spin density-modified heteroPOVs introduce different functionalities linked to the main-group metalloid atoms (E = Si, Ge, As, Sb) that may act as bonding mediators. The steric requirements around terminal V
O groups. Herein, we place emphasis on the structural variety of silicato-, germanato-, arsenato-, and antimonato-derivatised polyoxovanadates and their hybrid compounds with middle and late transition metals and discuss interesting molecular features and application perspectives of these heteroPOVs. Great interest in the polynuclear molecular vanadium oxides decorated with the heavier group 14 and 15 elements (E) is generated by the specific synthetic, structural, host–guest and vast redox chemistry and extraordinary magnetic characteristics of these compounds, involving e.g. geometrical spin-frustration.92 Remarkably, the structurally, electronically and spin density-modified heteroPOVs introduce different functionalities linked to the main-group metalloid atoms (E = Si, Ge, As, Sb) that may act as bonding mediators. The steric requirements around terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the heteroPOVs differ between the building blocks comprising E = SiIV, GeIV and those with E = AsIII, SbIII due to the presence of lone electron pairs at the latter elements. The fully-oxidised (VV), mixed-valent (VV/VIV and VIV/VIII), “fully-reduced” (VIV), and “highly-reduced” (VIII) heteroPOVs with various point-group symmetries and different isomeric structures of α and β types show an astonishing propensity for organic and transition metal/lanthanoid functionalization. This grants access to multifunctional inorganic–organic supramolecular materials93 and the closely-related chemistry of metal–organic frameworks (MOFs)94 and thus offers potential for applications of heteroPOVs in catalysis, surface science, and information technology.
O groups in the heteroPOVs differ between the building blocks comprising E = SiIV, GeIV and those with E = AsIII, SbIII due to the presence of lone electron pairs at the latter elements. The fully-oxidised (VV), mixed-valent (VV/VIV and VIV/VIII), “fully-reduced” (VIV), and “highly-reduced” (VIII) heteroPOVs with various point-group symmetries and different isomeric structures of α and β types show an astonishing propensity for organic and transition metal/lanthanoid functionalization. This grants access to multifunctional inorganic–organic supramolecular materials93 and the closely-related chemistry of metal–organic frameworks (MOFs)94 and thus offers potential for applications of heteroPOVs in catalysis, surface science, and information technology.
2. Group 14 (Si, Ge) element-functionalised POVs
2.1 Preview
The chemistry of heteroPOVs incorporating the most common {E2O7} groups and more rare {E2O5S2} ones with E = Si or Ge (SiPOVs and GePOVs) is still underdeveloped. In their molecular structures, the EIV atoms usually favour four-fold coordination, thus yielding tetrahedral {EO4} geometries. However, an example of a heteroPOV with a GeIV centre adopting a six-fold {GeO6} coordination was also described. Interestingly, the Si–O and Ge–O bond lengths (dE–O = 1.56–1.79 Å) are comparable to the bond lengths of terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (dV
O groups (dV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O = 1.56–1.64 Å), but shorter when compared to the single V–O bonds (dV–O = 1.88–2.28 Å). The {E2O7}- or {E2O5S2}-decorated POV structures are typically viewed as being formally derived from the [VIV18O42]12− archetype. The family of polyoxovanadatosilicates (SiPOVs) is represented by the assemblies with nuclearity V15, V17 and V18. The polyoxovanadatogermanates (GePOVs) display a series of assemblies classified as V6, V9, V12, V14, V15 and V16. The SiPOVs and GePOVs usually encapsulate discrete water molecules or halide ions and can exhibit negative charges up to 12−. The SiPOVs (Table 1) and GePOVs (Table 2) are usually synthesised by hydrothermal or solvothermal reactions using V2O5, VOSO4 and NH4VO3 as precursors.
O = 1.56–1.64 Å), but shorter when compared to the single V–O bonds (dV–O = 1.88–2.28 Å). The {E2O7}- or {E2O5S2}-decorated POV structures are typically viewed as being formally derived from the [VIV18O42]12− archetype. The family of polyoxovanadatosilicates (SiPOVs) is represented by the assemblies with nuclearity V15, V17 and V18. The polyoxovanadatogermanates (GePOVs) display a series of assemblies classified as V6, V9, V12, V14, V15 and V16. The SiPOVs and GePOVs usually encapsulate discrete water molecules or halide ions and can exhibit negative charges up to 12−. The SiPOVs (Table 1) and GePOVs (Table 2) are usually synthesised by hydrothermal or solvothermal reactions using V2O5, VOSO4 and NH4VO3 as precursors.
| Formula | Colour | Characteristics of crystal structurea | Reactants | Reaction conditions | Yield | Characterised via | Ref. |
|---|---|---|---|---|---|---|---|
| a Dimensionality resulting from hydrogen bonding networks is not considered. | |||||||
| SiPOV-based compounds | |||||||
| V15 | |||||||
| (H2pdn)3(Hpdn)[V15Si6O42(OH)6(Cl)]·nH2O (n = 7–10) | Brown | Close-packed layer aggregate | V2O5, H2O, pdn, tetraethyl orthosilicate (teos), HCl | 180 °C, 6 d | 72% based on V2O5 | EA, IR, TGA, EDXS, XPS, powder X-ray, single-crystal XRD, magnetometry | 98 |
| (H2pdn)(Hpdn)2[H6V15Si6O48][Co(pdn)2(H2O)]·9H2O | Red brown | 1D infinite chain | V2O5, pdn, H2O, teos, EtOH, Co(OAc)2·4H2O, HCl | 180 °C, 6 d | 35% based on V | EA, IR, XPS, single-crystal XRD, magnetometry | 96 |
| V17 | |||||||
| (H1.5pdn)6[{H2V15Si6O48(Cl)}(VO2)2]·4H2O | Brown | 1D zig-zag chain | V2O5, pdn, H2O, teos, EtOH, HOAc, HCl | 180 °C, 6 d | 83% based on V | EA, IR, XPS, single-crystal XRD, magnetometry | 96 |
| V17–18 | |||||||
| Cs10.5[(V16O40)(V1.5Si4.5O10)]·3.5H2O | Dark brown | 1D extended chain | VOSO4·3H2O, SiO2, H2O, CsOH | 240 °C, 3 d | EMP, IR, single-crystal XRD | 95 | |
| V18 | |||||||
| [H4V18O46(SiO)8(dab)4(H2O)]·4H2O | Dark green | Cross-linked 3D network | V2O5, SiO2, dab, H2O | 180 °C, 5 d | 61% based on SiO2 | EA, IR, powder XRD, single-crystal XRD | 97 |
| Formula | Colour | Characteristics of crystal structurea | Reactants | Reaction conditions | Yield | Characterised via | Ref. |
|---|---|---|---|---|---|---|---|
| a Dimensionality resulting from hydrogen bonding networks is not considered. | |||||||
| V6 | |||||||
| [V6Ge5O21(heda)6]·3H2O | Pale purple | Layer-like arrangement | NH4VO3, GeO2, Hheda, H2O | 160 °C, 3 d | 65% based on NH4VO3 | EA, IR, EPR, TGA, powder XRD, single-crystal XRD, optical diffuse reflection | 109 |
| V9 | |||||||
| (NH4)2[H2V9Ge6O26(L)6]·0.65H2O | Blue | NH4VO3, GeO2, H2O, HF, ethylene glycol (H2L) | 170 °C, 3 d | 58% based on Ge | EA, IR, TGA, EPR, single-crystal XRD | 103 | |
| V12 | |||||||
| K5[H8V12Ge8O48(SO4)]·10H2O | Brown | Channels | VOSO4, GeO2, KOH | 180 °C, 4 d | IR, single-crystal XRD | 100 | |
| [Cd(en)2]2[Cd2(en)2V12O40(GeOH)8(H2O)]·6H2O | Black | 1D sinusoidal chain | NH4VO3, GeO2, CdCl2·2.5H2O, H3BO3, en, H2O | 170 °C, 5 d | 80% based on GeO2 | EA, IR, TGA, EDXS, powder XRD, single-crystal XRD, magnetometry | 110 |
| V14 | |||||||
| K2Na6[V14GeO40]·10H2O | Blue black | NaVO3, GeBr2, NaOAc, KCl | 50 °C (1 h) → r.t. | 64% | EA, IR, UV-vis, 51V-NMR, XPS, electrochemistry, EPR, single-crystal XRD, magnetometry | 104 | |
| (H2ppz)4(Hppz)4[V14Ge8O50(H2O)] | Olive brown | VOSO4, GeO2, ppz, H2O | 170 °C, 4 d | 45% based on V | EA, IR, TGA, single-crystal XRD | 100 | |
| (H2ppz)4(Hppz)4[V14Ge8O50(H2O)] | Black | NH4VO3, GeO2, Cu(NO3)2·3H2O, aep, H2O | >150 °C, 9 d | EA, single-crystal XRD | 101 | ||
| (H3aep)4[V14Ge8O50]·2(aep)·13H2O | Dark brown | Layer-like arrangement | NH4VO3, GeO2, Cu(NO3)2·3H2O, aep, H2O | <150 °C, 9 d | 73% based on GeO2 | EA, IR, DTA-TG, single-crystal XRD, magnetometry | 101 |
| (H2dab)4[V14O44(GeOH)8]·6H2O | Dark green | V2O5, GeO2, dab, H2O | 170 °C, 5 d | 69% based on GeO2 | EA, IR, single-crystal XRD | 97 | |
| (H3dien)4[V14Ge8O42S8]·5H2O | Black | Layer-like arrangement | NH4VO3, Ge, S, dien, H2O | 160 °C, 7 d | 60% based on Ge | EA, DTA-TG-MS, powder X-ray, single-crystal XRD | 99 |
| (H3aep)4[V14Ge8O42S8] | Black | Layer-like arrangement | NH4VO3, Ge, S, aep, H2O | 170 °C, 7 d | 60% based on Ge | EA, DTA-TG-MS, powder X-ray, single-crystal XRD, magnetometry | 99 |
| [{Cd(en)}4V10Ge8O46(H2O)[V(H2O)2]4(GeO2)4]·8H2O | Red | 3D framework | NH4VO3, GeO2, CdCl2·2.5H2O, H3BO3, en, H2O | 170 °C, 4 d, pH = 9.7–10 | 36% based on GeO2 | EA, IR, EDXS, XPS, TGA, powder XRD, single-crystal XRD, magnetometry | 112 |
| [{Cd(enMe)}4V10Ge8O46(H2O)[V(H2O)2]4(GeO2)4]·8H2O | Red | 3D framework | NH4VO3, GeO2, CdCl2·2.5H2O, H2C2O4·2H2O, enMe, H2O | 170 °C, 4 d, pH = 9.7–10 | 52% based on GeO2 | EA, IR, EDXS, XPS, TGA, powder XRD, single-crystal XRD, magnetometry | 112 |
| V15 | |||||||
| (H2tren)2(H3tren)[V15Ge6O42(OH)6(Cl)]·2H2O | Brown | 1D channel | NH4VO3, GeO2, H2O, tren, HCl | 170 °C, 5 d | 53% based on NH4VO3 | EA, IR, TGA, EDXS, XPS, powder X-ray, single-crystal XRD | 98 |
| [Zn2(enMe)3][Zn(enMe)]2[V15Ge6O48(H2O)][Zn(enMe)2(H2O)]2·3H2O | Brown | 2D layered network | V2O5, GeO2, ZnSO4, enMe, H2O | 170 °C, 3 d | 78% based on GeO2 | EA, IR, TGA, EDXS, powder XRD, single-crystal XRD, magnetometry | 110 |
| [{Ni(tren)}4(H2tren)2V15Ge6O48(H2O)]·2H2O | Dark brown | 2D layer | NH4VO3, GeO2, Ni(NO3)2·6H2O, tren, H2O | 130 °C, 7 d | 43% based on V | EA, IR, TGA, powder XRD, single-crystal XRD, magnetometry | 114 |
| [Co(tren)(H2tren)]2[{Co(tren)}2V15Ge6O42S6(H2O)]·9H2O | Dark red brown | Channels | NH4VO3, Ge, Co, S, tren, H2O | 140 °C, 7 d | 60% based on V | EA, IR, TG-DTA-MS, single-crystal XRD, magnetometry | 102 |
| [{Mn(tren)(H2tren)}{Mn(tren)}4V15Ge6O48(H2O)0.5]·(tren)·2H2O | Dark brown | Helical 1D strand | NH4VO3, GeO2, MnCl2·2H2O, tren, H2O | 130 °C, 7 d | 41% based on V | EA, IR, TGA, powder XRD, single-crystal XRD, magnetometry | 114 |
| V16 | |||||||
| Cs8[V16Ge4O42(OH)4]·4.7H2O | Brown | Layer-like arrangement | VOSO4, GeO2, H2O, CsOH | 170 °C, 3 d | 30% based on V | IR, EMP, TGA, single-crystal XRD | 100 |
| [Cd3(dien)2(Hdien)2(H2O)2][V16Ge4O42(OH)4(H2O)]·2H2O | Black | 3D open framework | V2O5, GeO2, CdCl2·2.5 H2O, dien, ethylene glycol, H2O | 170 °C, 3 d | 90% based on GeO2 | EA, IR, TGA, EDXS, powder XRD, single-crystal XRD, magnetometry | 110 |
| [Co(enMe)2]3[Co2(enMe)4][V16Ge4O44(OH)2(H2O)]·5H2O | Brown | 3D open framework | V2O5, GeO2, Co(OAc)2·4H2O, H2O, enMe | 170 °C, 5 d | 86% based on V | EA, IR, XPS, TGA, powder XRD, single-crystal XRD, magnetometry | 113 |
| [Co2(en)3][Co(en)2]2[Co(en)2(H2O)][V16Ge4O44(OH)2(H2O)]·10.5H2O | Brown | 3D open framework | V2O5, GeO2, Co(OAc)2·4H2O, H2O, en | 170 °C, 5 d | 63% based on V | EA, IR, XPS, TGA, powder XRD, single-crystal XRD, magnetometry | 113 |
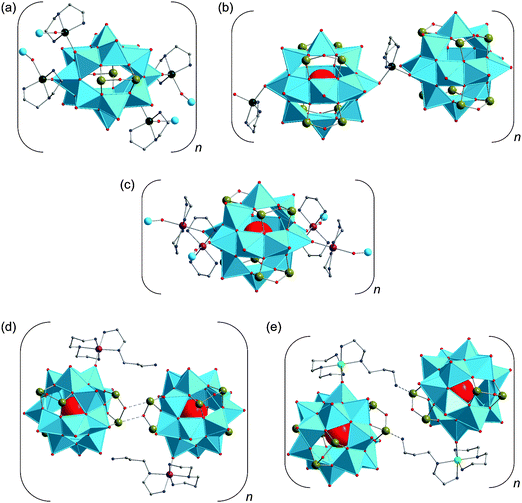
2.2 Polyoxovanadatosilicates (SiPOVs)
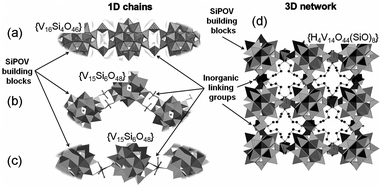
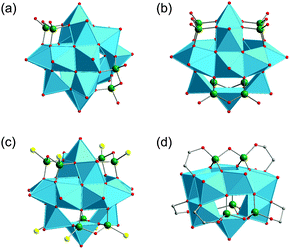
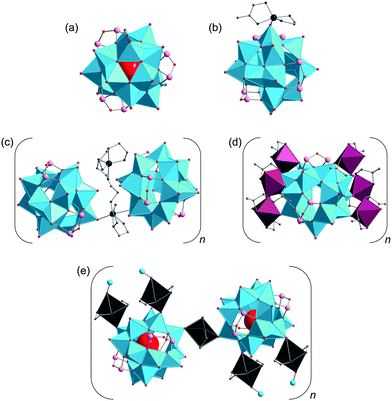
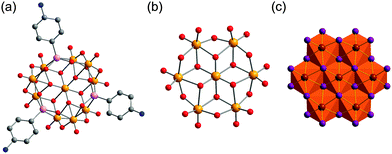
Only a few studies describing the isolation of SiPOVs have been performed so far. These SiPOVs constitute a series with the general formula {V18−zSi2zO42+2z} and are characterised by antiferromagnetic coupling between the spin-1/2 vanadyl {VO}2+ moieties.Vanadyl(V)-extended SiPOVs. The first report about the spherical POV chemically modified with SiIV dates back to 2001 when Jacobson and coworkers described the [VIV16Si4O46]12− polyoxoanion (Fig. 6a) that is a component of the compound Cs10.5[(V16O40)(V1.5Si4.5O10)]·3.5H2O synthesised under hydrothermal conditions (Table 1).95 The crystal structure of this hybrid compound shows a 1D linear chain of the “fully-reduced” [VIV16O40]16− POVs interlinked by vanadosilicate six-membered rings with the composiiton {VV1.5Si4.5O10}5.5+ (Fig. 7a). [V16O40]16− is hypothetically derived from the α-Keggin {V12O36} archetype or, accordingly, the {V18O42} archetype (Fig. 4). Replacing two vanadyl {VO}2+ groups on two opposite sides of the [V18O42]12− isopolyoxoanion by two silicate {Si2O3} subunits, the spherical [VIV16Si4O46]12− dodecaanion is formed. A water molecule is located in the void of the polyoxoanion.
Later on, two extended chains based on the “fully-reduced” {VIV15Si6O48} building blocks96 were obtained from reactions under hydrothermal conditions where the pdn molecules (pdn = 1,3-propanediamine) acted as reductant for VV (Table 1). These two inorganic–organic hybrid compounds can be assigned to the classes of vanadyl-extended and transition metal complex (TMC)-supported SiPOVs.
1D zig-zag chains of the SiPOVs doubly bridged by the fully-oxidised {VVO2}+ moieties through (Si–)O–V–O(–Si) bonds with dV–O = 1.79 Å and 1.81 Å (Fig. 7b) were observed in the crystal of the compound (H1.5pdn)6[{H2V15Si6O48(Cl)}(VO2)2]·4H2O.96 The partially protonated pdn molecules compensate the high negative charge of the mixed-valent [{Cl@H2VIV15Si6O48}(VVO2)2]9− assembly and reside in the voids between the zig-zag chains, along with H2O solvate molecules.
TMC-supported SiPOV. One-dimensional infinite chains of [H6VIV15Si6O48]6− polyoxoanions that are singly bridged by [Co(pdn)2(H2O)]2+ fragments through (V–)O–Co–O(–Si,V) linkages (Fig. 7c) were found in the crystal structure of (H2pdn)(Hpdn)2[H6V15Si6O48][Co(pdn)2(H2O)]·9H2O.96
2.3 Polyoxovanadatogermanates (GePOVs)
The compound (H2dab)4[V14O44(GeOH)8]·6H2O was obtained by Clearfield and coworkers from the hydrothermal reaction between V2O5, GeO2 and 1,4-diaminobutane (dab) in water (Table 2).97 The “fully-reduced”, protonated α-[VIV14O44(GeOH)8]8− polyoxoanion is isostructural to the aforementioned α-[H4VIV14O44(SiO)8]12− polyoxoanion (Fig. 6b). In contrast to [H4V18O46(SiO)8(dab)4(H2O)]·4H2O, the Ge(IV)-containing compound contains doubly protonated H2dab2+ countercations.
A GePOV structural analogue to the [Cl@VIV15Si6O42(OH)6]7− polyoxoanion (Fig. 6c) was also reported. The main structural difference between the two compounds containing the [Cl@V15E6O42(OH)6]7− component is the packing of the structural building blocks. The compound with E = Si shows a close-packed layer structure, whereas the one with E = Ge exhibits a 1D chain structure.98 The crystal structure of the GePOV-based compound (Table 2) with the formula (H2tren)2(H3tren)[V15Ge6O42(OH)6(Cl)]·2H2O is furthermore characterised by the N–H⋯O hydrogen bonds formed between the oxygen atoms of the “fully-reduced” polyoxoanions and the organic tris(2-aminoethyl)amine (tren) countercations.
The compound (H3aep)4[V14Ge8O50]·2(aep)·13H2O (aep = 1-(2-aminoethyl)piperazine = C6H15N3) and the already described (H2ppz)4(Hppz)4[V14Ge8O50(H2O)] compound100 with the “fully-reduced” α-[VIV14Ge8O50]12− components were formed in solvothermal reactions involving Cu(NO3)2·3H2O below 150 °C and above 150 °C, respectively (Table 2).101 Since Cu2+ was reduced to metallic Cu during the syntheses, the linking of the GePOV building blocks through Cu2+-centred complexes into extended structures was unsuccessful. Interestingly, (H3aep)4[V14Ge8O50]·2(aep)·13H2O could also be produced without utilising the copper salt Cu(NO3)2·3H2O. However, the presence of copper in the reaction mixture turned out to be crucial for the crystallisation of (H2ppz)4(Hppz)4[V14Ge8O50(H2O)]. The high negative charge of [VIV14Ge8O50]12− (Fig. 8b) is compensated by the protonated amine molecules. Weak (>2 Å) and strong (<2 Å) N–H⋯O hydrogen bonds between the organic ammonium countercations and the exposed oxygen atoms of the GePOVs in the crystals of both compounds result in 3D H-bonded networks. The preliminary analysis of the magnetic properties of (H3aep)4[V14Ge8O50]·2(aep)·13H2O indicated strong antiferromagnetic exchange interactions between the spin-1/2 vanadyl {VO}2+ moieties through the near-linear V–μ-O–V and bent V(–μ-O–)2V bridges.
Discrete GePOV. The structural diversity of the GePOVs was explored towards their sulfur-functionalised analogues. Two polyoxothio compounds (H3dien)4[V14Ge8O42S8]·5H2O (dien = diethylenetriamine) and (H3aep)4[V14Ge8O42S8] consisting of the “fully-reduced” [VIV14Ge8O42S8]12− GePOVs and protonated amines as countercations were synthesised hydrothermally (Table 2).99 The α-[VIV14Ge8O42S8]12− polyoxoanion (Fig. 8c) with a diameter of ca. 7.4 Å shows structural, but not functional similarities to α-[VIV14Ge8O50]12− (Fig. 8b). The {V14}-nuclearity building block of the thio-modified GePOV is composed of fourteen condensed {VO5} square pyramids and eight tetrahedral {GeO3S} units. Thus, eight terminal oxygens sites of four {Ge2O7} groups were formally exchanged for eight sulfur atoms to form four {Ge2O5S2} groups.
Cobalt–GePOV hybrid. Another example of thio-functionalised GePOV, [H2O@VIV15Ge6O42S6]12−, composed of six slightly distorted {GeO3S} tetrahedra (dGe–S = 2.09–2.14 Å), fifteen {VO5} square pyramids and an encapsulated central H2O molecule was obtained under hydrothermal conditions using metavanadate as precursor and elemental Ge, Co and S (Table 2).102 The spherical [VIV15Ge6O42S6]12− structure is constructed of three {V7Ge2O24S2} rings which are perpendicular to each other. This polyoxoanion is formally derived from the {V18O42} archetype by substituting three {VO5} pyramidal units of [V18O42]12− by three handle-like {Ge2O5S2} groups (for comparison, see [VIV15Si6O42(OH)6]6− in Fig. 6c). This “fully-reduced” GePOV is further expanded by two {Co(tren)}2+ complexes through Co–S bonds, thus featuring the Co–S
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ge–O–V connections. These two trigonal-bipyramidal Co(II) complexes are attached to the terminal sulfide sites (S2−) and, along with two [Co(tren)(H2tren)]4+ countercations, reduce the high negative charge of the polyoxoanion to give the compound [Co(tren)(H2tren)]2[{Co(tren)}2V15Ge6O42S6(H2O)]·9H2O where tren molecules act as mono- and tetradentate ligands. The presence of the N–H⋯O hydrogen-bonding pattern in the crystal structure of this compound results in two differently oriented channels. Similar to other {VIV15E6}-type structures (see also Section 3.2.5), the magnetic structure of the GePOV building block can be described as being composed of a geometrically frustrated, antiferromagnetically coupled equilateral {V3} triangle that is sandwiched between the two {V6} hexagons characterised by strong antiferromagnetic nearest-neighbor coupling.
Ge–O–V connections. These two trigonal-bipyramidal Co(II) complexes are attached to the terminal sulfide sites (S2−) and, along with two [Co(tren)(H2tren)]4+ countercations, reduce the high negative charge of the polyoxoanion to give the compound [Co(tren)(H2tren)]2[{Co(tren)}2V15Ge6O42S6(H2O)]·9H2O where tren molecules act as mono- and tetradentate ligands. The presence of the N–H⋯O hydrogen-bonding pattern in the crystal structure of this compound results in two differently oriented channels. Similar to other {VIV15E6}-type structures (see also Section 3.2.5), the magnetic structure of the GePOV building block can be described as being composed of a geometrically frustrated, antiferromagnetically coupled equilateral {V3} triangle that is sandwiched between the two {V6} hexagons characterised by strong antiferromagnetic nearest-neighbor coupling.
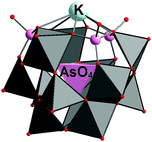
GePOV with a {GeO6} group. A mixed-valent [VIV2VV12GeO40]8− polyoxoanion with an octahedrally coordinated GeIV heteroatom occupying a central geometrical position within the GePOV structure was isolated as the compound K2Na6[V14GeO40]·10H2O.104 The compound was obtained by reaction of GeBr2 and NaVO3 in aqueous acidic solution where GeBr2 acted as a reducing agent for the VV source (Table 2). This D4h-symmetric GePOV consists of a central {GeIVO6} octahedron and fourteen {VO6} octahedra, all of which are edge-shared (Fig. 9). Interestingly, this polyoxoanion is the structural analogue to the [VIV2VV12AlIIIO40]9−
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 and [VIV2VV12AsVO40]7−
105 and [VIV2VV12AsVO40]7−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 polyoxoanions; the Al-containing heteroPOV is found in the mineral sherwoodite, Ca4.5[V14AlO40]. It is worth mentioning that the [VIV2VV12GeO40]8− polyoxoanion extends the family of heteroPOVs where the main-group heteroatoms (E = AlIII, GeIV, AsV) reside within the {V14O40} cores. The dicapped cuboctahedral topology was ascribed to the {VIV2VV12EO40} building blocks. Magnetic studies of K2Na6[V14GeO40]·10H2O showed that two apical spin-1/2 VIV ions, which are separated from each other by 8.52 Å via one O–Ge–O bond, are weakly antiferromagnetically coupled. X-band and Q-band EPR measurements indicated very weak spin–spin exchange coupling between these VIV ions. The magnetic properties of the mixed-valent [V14GeO40]8− were theoretically studied by Suaud, Coronado and coworkers on the basis of model Hamiltonian calculations and wave function theory as well as an electric field approach.107 In particular, it was shown that an external electric field can induce a reversible transition from a paramagnetic (triplet) to an antiferromagnetic (singlet) ground state configuration of [V14GeO40]8−, meaning that this polyoxoanion can in principle act as a molecular switch. For a discussion about the relevance of [V14GeO40]8− to the field of molecular spintronics, see the review by Coronado and coworkers.75 Moreover, the [VIV2VV12GeO40]8− polyoxoanion was reported to be a two-electron catalyst for the oxidation of the coenzyme NADH at pH = 8. Note that POMs usually act as one-electron catalysts.108
106 polyoxoanions; the Al-containing heteroPOV is found in the mineral sherwoodite, Ca4.5[V14AlO40]. It is worth mentioning that the [VIV2VV12GeO40]8− polyoxoanion extends the family of heteroPOVs where the main-group heteroatoms (E = AlIII, GeIV, AsV) reside within the {V14O40} cores. The dicapped cuboctahedral topology was ascribed to the {VIV2VV12EO40} building blocks. Magnetic studies of K2Na6[V14GeO40]·10H2O showed that two apical spin-1/2 VIV ions, which are separated from each other by 8.52 Å via one O–Ge–O bond, are weakly antiferromagnetically coupled. X-band and Q-band EPR measurements indicated very weak spin–spin exchange coupling between these VIV ions. The magnetic properties of the mixed-valent [V14GeO40]8− were theoretically studied by Suaud, Coronado and coworkers on the basis of model Hamiltonian calculations and wave function theory as well as an electric field approach.107 In particular, it was shown that an external electric field can induce a reversible transition from a paramagnetic (triplet) to an antiferromagnetic (singlet) ground state configuration of [V14GeO40]8−, meaning that this polyoxoanion can in principle act as a molecular switch. For a discussion about the relevance of [V14GeO40]8− to the field of molecular spintronics, see the review by Coronado and coworkers.75 Moreover, the [VIV2VV12GeO40]8− polyoxoanion was reported to be a two-electron catalyst for the oxidation of the coenzyme NADH at pH = 8. Note that POMs usually act as one-electron catalysts.108
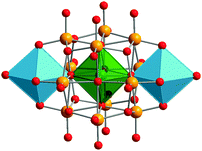 | ||
| Fig. 9 Structure of the mixed-valent [VIV2VV12GeO40]8− polyoxoanion, as present in K2Na6[V14GeO40]·10H2O. Colour code: O, red; Ge, green; VIVOx, sky-blue polyhedra; VV, light orange. | ||
Neutral GePOV. Another two-electron reduced GePOV was reported by Bian, Dai and colleagues.109 The mixed-valent compound [VIV2VV4Ge5O21(heda)6]·3H2O, in which the deprotonated amine ligands [Hheda = N-(2-hydroxyethyl)ethylenediamine] coordinate covalently to the GePOV shell in three different coordination modes (monodentate and chelating for V centres, and multi-chelating for Ge centres), was obtained by solvothermal synthesis using Hheda as a reducing agent for VV (Table 2). The pale purple colour of this low-nuclearity compound was shown to be due to the absorption bands at 2.18 eV (568 nm) and 1.46 eV (856 nm) assigned to d–d transitions of the vanadium ions in quasi-octahedral coordination. The central fragment of [V6Ge5O21(heda)6]·3H2O is a C2 symmetrical GePOV, which is not structurally related to the {V18O42} archetype like the above-mentioned “fully-reduced” alkoxoGePOV [H2VIV9Ge6O26(L)6]2−
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 and the mixed-valent [VIV2VV12GeO40]8− polyoxoanion.104 The structure of the neutral [V6Ge5O21(heda)6] compound is composed of two {VO5} square pyramids, two {VO6} octahedra and two {VO5N} octahedra and involves three tetrahedral {GeO4} and two octahedral {GeO4N2} moieties. According to the optical diffuse reflectance spectrum, [V6Ge5O21(heda)6]·3H2O possesses an energy gap of 2.88 eV.
103 and the mixed-valent [VIV2VV12GeO40]8− polyoxoanion.104 The structure of the neutral [V6Ge5O21(heda)6] compound is composed of two {VO5} square pyramids, two {VO6} octahedra and two {VO5N} octahedra and involves three tetrahedral {GeO4} and two octahedral {GeO4N2} moieties. According to the optical diffuse reflectance spectrum, [V6Ge5O21(heda)6]·3H2O possesses an energy gap of 2.88 eV.
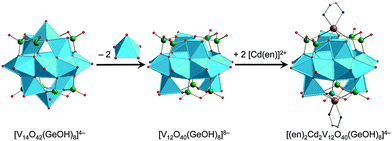
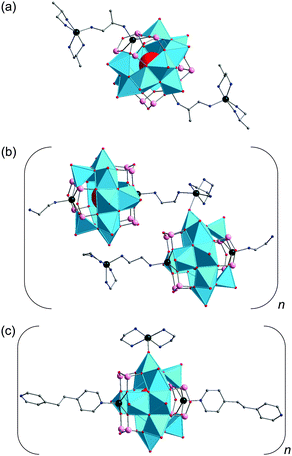
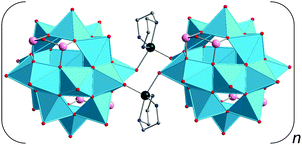
Zinc– and cadmium–GePOV hybrids. Yang and coworkers succeeded in the hydrothermal synthesis of a series of the inorganic–organic hybrid materials [Cd(en)2]2[Cd2(en)2V12O40(GeOH)8(H2O)]·6H2O (en = ethylenediamine = C2H8N2), [Zn2(enMe)3][Zn(enMe)]2[V15Ge6O48(H2O)][Zn(enMe)2(H2O)]2·3H2O (enMe = 1,2-diaminopropane), and [Cd3(dien)2(Hdien)2(H2O)2][V16Ge4O42(OH)4(H2O)]·2H2O (Table 2).110 The adjustment of the pH value to the alkaline media (pH = 8.8–12.5) was crucial for isolation of these products. Their crystal structures show the “fully-reduced” polyoxoanions [Cd2(en)2VIV12O40(GeOH)8(H2O)]4− (Fig. 10), [H2O@VIV15Ge6O48]12− and [H2O@VIV16Ge4O42(OH)4]8− (Fig. 8a) which are interconnected by transition metal cations [Cd(en)2]2+, [Zn(enMe)]2+/[Zn2(enMe)3]4+ and [Cd3(μ-dien)2(Hdien)2(H2O)2]8+, respectively. These bind to the GePOV building blocks through the O atoms of {VO5} square pyramids or the apical O atoms of {GeO4} tetrahedra, thus forming Cd–Oterm–V and Zn–Oterm–Ge connectivities. Interestingly, the [Cd2(en)2VIV12O40(GeOH)8]4− polyoxoanion illustrated in Fig. 10 incorporates two [Cd(en)]2+ units in the dilacunary-type α-[VIV12O40(GeOH)8]8− backbones. The 1D sinusoidal chains established for [Cd(en)2]2[Cd2(en)2V12O40(GeOH)8(H2O)]·6H2O with dV⋯V = 2.86–3.03 Å are additionally arranged through N–H⋯O and O–H⋯O hydrogen bonds to afford a 3D supramolecular framework with pseudocircular channels. The interchain distance was found to be ca. 10.65 Å. The {V15}- and {V16}-nuclearity GePOVs are the representatives of the class of polynuclear molecular mixed-metal oxides with the general formula [V18−zGe2zO42(O,OH)2z] (z = 2, 3) and show interesting structural relationships to other reported GePOVs and SiPOVs. Whereas [VIV15Ge6O48]12− and [VIV15Si6O42(OH)6]6− (Fig. 6c) are isostructural analogues, the polyoxoanions [VIV16Ge4O42(OH)4]8− (Fig. 8a) and [VIV16Si4O46]12− (Fig. 6a) are conformational isomers differing by the geometrical positions of the handle-like {E2O7} groups in the POV structure. While the [Cd2(en)2VIV12O40(GeOH)8(H2O)]4− and [VIV15Ge6O48]12− polyoxoanions exhibit strong antiferromagnetic coupling, the magnetic behaviour of the [VIV16Ge4O42(OH)4]8− polyoxoanion was reported to exhibit intramolecular ferrimagnetic coupling, which is rare for such POMs.111
Tetra-CdII-substituted GePOVs were also reported. Two isomorphic, mixed-valent compounds with the general formula [{CdL}4V10Ge8O46(H2O)[V(H2O)2]4(GeO2)4]·8H2O (L = en, enMe) were synthesised under hydrothermal conditions using the weak acids H3BO3 or H2C2O4·2H2O and the alkaline media at pH = 9.7–10 (Table 2).112 Their crystal structures exhibit 3D 10-connected, inorganic–organic frameworks (point-symbol of 312·428·55 for the bct topology) which are constructed from the D4h-symmetric, “fully-reduced” [{CdR}4VIV10Ge8O46(H2O)]12− hybrid polyoxoanions covalently connected to each other via the planar, “highly-reduced” [VIII4O2(H2O)8]8+ units (dV⋯V = 2.47 Å) and the bridging {GeO4} tetrahedra (an unusual situation in POM chemistry, however observed for {SiO4} units in Cs10.5[(V16O40)(V1.5Si4.5O10)]·3.5H2O95). The Cd2+ ions are incorporated into the backbones of the tetralacunary β-isomeric GePOV building block composed of ten {VO5} square pyramids and thus display trigonal prismatic geometries (CdO4N2), similarly to the “fully-reduced” [Cd2(en)2VIV12O40(GeOH)8]4− hybrid polyoxoanion (Fig. 10). An attempt to replace these Cd2+ ions coordinated in a circular {{CdR}4Ge8O28}16− constituent of the dodecaanion by Mn2+, Fe2+, Co2+, Ni2+ or Zn2+ under applied reaction conditions (Table 2) has failed. This type of CdII-substituted GePOVs exhibit antiferromagnetic properties.
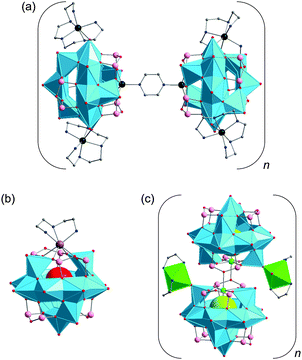
Cobalt–GePOV hybrids. Further two {V16Ge4}-type polyoxoanions now supported by bridging CoII complexes were described by Xu, Hu and colleagues.113 The compounds [Co(enMe)2]3[Co2(enMe)4][V16Ge4O44(OH)2(H2O)]·5H2O and [Co2(en)3][Co(en)2]2[Co(en)2(H2O)][V16Ge4O44(OH)2(H2O)]·10.5H2O with extended 3D frameworks in their crystal structures were obtained hydrothermally in alkaline solutions at pH = 10.2–10.8 (Table 2). The structures of these compounds consist of the “fully-reduced” [H2O@VIV16Ge4O44(OH)2]10− anions charge-balanced by Co2+ centred amine complexes and exhibit different structural topologies. Whereas [Co(enMe)2]3[Co2(enMe)4][V16Ge4O44(OH)2(H2O)]·5H2O adopts a NaCl-type network composed of different TMC linkers and [H2O@VIV16Ge4O44(OH)2]10− polyoxoanions as 6-connected nodes, [Co2(en)3][Co(en)2]2[Co(en)2(H2O)][V16Ge4O44(OH)2(H2O)]·10.5H2O is characterised by a (4,6)-connected network [Schläfli symbol (46·67·82)2(42·64)], which was previously not known for heteroPOVs. Two types of relatively short interatomic distances dV⋯V = 2.80–3.07 Å and dCo⋯V = 3.38–3.60 Å were observed. According to the variable temperature susceptibility measurements, these two compounds feature ferrimagnetic characteristics.
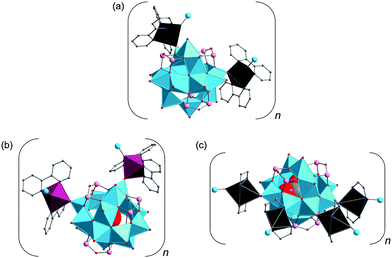
Manganese– and nickel–GePOV hybrids. The solvothermally prepared [{Mn(tren)(H2tren)}{Mn(tren)}4V15Ge6O48(H2O)0.5]·tren·2H2O and [{Ni(tren)}4(H2tren)2V15Ge6O48(H2O)]·2H2O compounds are characterised by the {V15Ge6}-type polyoxoanions which could be expanded into the high-nuclearity GePOV networks.114 The structural analysis of these charge-neutral helical 1D strands and 2D layers revealed different coordination environments of unique, in situ-formed MnII and NiII complexes as well as different interconnection modes of the spherical, “fully-reduced” [VIV15Ge6O48]12− polyoxoanions with encapsulated water guest molecules. Interestingly, the reaction conditions were very similar in each case except the nature and amount of transition metal starting materials (Table 2). The central {V15Ge6O48(H2O)0.5} structural motif of [{Mn(tren)(H2tren)}{Mn(tren)}4V15Ge6O48(H2O)0.5]·tren·2H2O, which is stable up to 350 °C, is coordinated by five crystallographically independent MnII complexes via Mn–O(–V/Ge) bonds. The compound exhibits a three layer solid-state structure, which is composed of the following subfragments: (i) six corner- and edge-sharing square pyramidal {VO5} units and a [MnO(H2tren)(tren)]2+ complex; (ii) a {V3Ge6O28} ring, [Mn(tren)]2+ and [Mn2O2(tren)2]4+ complexes; (iii) six corner- and edge-sharing {VO5} square pyramids. The crystal structure of this GePOV-based inorganic–organic hybrid displays [Mn(tren)]2+ and [Mn(tren)(H2tren)]4+ moieties and a rare dinuclear [Mn2O2(tren)2]4+ fragment that bridges two terminal O atoms of the handle-like {Ge2O7} groups from the adjacent GePOVs. In contrast to [Mn2O2(tren)2]4+, the [Mn(tren)(H2tren)]4+ complex is bound to the surface of the GePOV shell via a terminal O atom of a pyramidal {VO5} unit. The crystal structure of [{Ni(tren)}4(H2tren)2V15Ge6O48(H2O)]·2H2O comprises binuclear [{Ni(tren)}(H2tren){Ni(tren)}]6+ complexes, which coordinate only to the {VO5} square pyramids of the polyoxoanion and bridge the neighbouring {V15Ge6O48} building blocks through V
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–Ni bonds in a manner to allow for the formation of “rhombic windows” in which crystal water molecules reside. The magnetochemical analysis of these two compounds indicated that neither MnII nor NiII ions affect the spin structure of the [V15Ge6O48]12− polyoxoanion showing the typical “V6–V3–V6” layer structure with V⋯V distances ranging from 2.84 to 3.10 Å. The compounds feature weak antiferromagnetic interactions between the spin-1/2 vanadyl {VO}2+ moieties of the {V15Ge6O48} building block and the adjoined MnII or NiII complexes.
O–Ni bonds in a manner to allow for the formation of “rhombic windows” in which crystal water molecules reside. The magnetochemical analysis of these two compounds indicated that neither MnII nor NiII ions affect the spin structure of the [V15Ge6O48]12− polyoxoanion showing the typical “V6–V3–V6” layer structure with V⋯V distances ranging from 2.84 to 3.10 Å. The compounds feature weak antiferromagnetic interactions between the spin-1/2 vanadyl {VO}2+ moieties of the {V15Ge6O48} building block and the adjoined MnII or NiII complexes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (Fig. 8c) and [H2O@VIV15Ge6O42S6]12−
99 (Fig. 8c) and [H2O@VIV15Ge6O42S6]12−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102 polyoxoanions. The {V16Ge4}-type GePOVs were found to exhibit ferrimagnetic properties.110,113
102 polyoxoanions. The {V16Ge4}-type GePOVs were found to exhibit ferrimagnetic properties.110,113
3. Group 15 (As, Sb) element-functionalised POVs
3.1 Preview
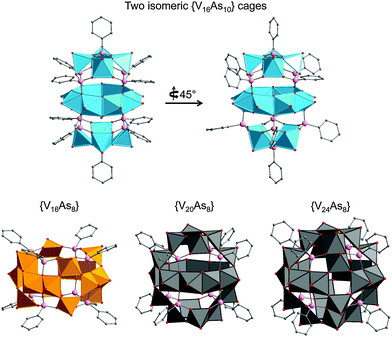
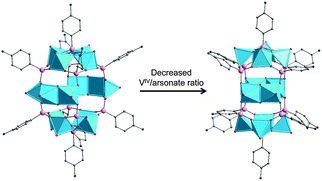
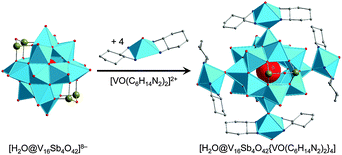
One of the key differences between the SiPOVs/GePOVs and the polyoxovanadatoarsenates (AsPOVs)/polyoxovanadatoantimonates (SbPOVs) is the absence of the effective electron lone pairs at SiIV/GeIV in the former and their presence at AsIII/SbIII in the latter. Note that these electron lone pairs provide different steric hindrances around the E and terminal oxygen atoms (Oterm) in the heteroPOVs, thus influencing the reactivity of the E sites towards the TMC complexes and organic ligands. In comparison to SiPOVs and GePOVs, the chemistry of Sb- and, especially, As-incorporating POVs is more widely developed and resulted in a variety of discrete and multidimensional structures with interesting chemical and physical properties. The AsPOVs and SbPOVs are frequently prepared under hydrothermal or solvothermal conditions (Tables 3 and 4). The AsPOVs known thus far are characterised by both the low-nuclearity and high-nuclearity assemblies, which are classified as V5, V6, V10, V12, V13, V14, V15, V16, V20 and V24 series. SbPOVs show assemblies with nuclearity V14, V15, V16 and V20. Some of their most common skeletons are shown in Fig. 11.| Formula | Colour | Characteristics of crystal structurea | Reactants | Reaction conditions | Yield | Characterised via | Ref. |
|---|---|---|---|---|---|---|---|
| a Dimensionality resulting from hydrogen bonding networks is not considered. | |||||||
| V5 | |||||||
| Na5[V5O9(O3AsC6H4-4-NH2)4]·20.5H2O·3DMF | Green | NaVO3, NaN3, p-arsanilic acid, H2O, DMF, N2H4·H2O, HCl | 70 °C, pH = 7.3 | 82% | EA, IR, UV-vis, single-crystal XRD | 166 | |
| V6 | |||||||
| (NnBu4)4[V6As8O26] | Bright green | NaVO3, H2O, NaAsO2, N2H4, nBu4NBr | r.t. | 50% based on As | EA, IR, TGA, single-crystal XRD, magnetometry | 137 | |
| (NnBu4)2[H2{V6O10(O3AsPh)6}]·2H2O | Dark green | PhAsO3H2, (NH4)2Na2K2[V10O28], (TBA)Br, MeOH, MeCN, pentane, Et2O, isopropanol | Reflux, 40 min → 4 d | 65% | EA, IR, UV-vis, electrochemistry, magnetometry, single-crystal XRD | 161 | |
| V10 | |||||||
| [V10As2O26(H2O)]·8H2O | Dark green | 3D network | V2O5, H3AsO4, H2C2O4, H2O, en | 160 °C, 3 d, pH = 8 | 40% based on V | EA, IR, TGA, EPR, powder XRD, single-crystal XRD, magnetometry | 118 |
| H5[V10O18(O3AsC6H4-4-NH2)7(DMF)2]·7DMF·5H2O | Green | Hexagonal packing arrangement | NaVO3, (p-aminophenyl)arsonic acid, H2O, DMF, HNO3, N2H4·H2O | 70 °C, 30 min, pH = 4 | 35% | EA, IR, DTA-TG, powder XRD, single-crystal XRD | 165 |
| (NnBu4)2(NH4)2[V10O24(O3AsC6H4-4-NH2)3] | Red | (TBA)Br, arsanilic acid, MeOH, (NH4)2Na2K2[V10O28] | Reflux, 20 min → 4 °C, 20 h | 45% | EA, IR, UV-vis, 51V NMR, EPR, electrochemistry, single-crystal XRD | 161 | |
| V12 | |||||||
| (NH4)4[V12As8O40(H2O)]·4H2O | Dark green | NH4VO3, As2O3, ethyl ether (or EtOH), H2O | 80 °C, 7 d | 20% based on V | EA, IR, ESI-MS, TG-MS, UV-vis, powder XRD, single-crystal XRD | 122 | |
| (NHEt3)4[V12As8O40(H2O)]·H2O | Dark blue | NaVO3, As2O3, NHEt3Cl, H2O, N2H4·HCl, HCl | 24 °C, 2–3 d, pH = 6.0 | 29% based on V | IR, magnetometry, powder XRD, single-crystal XRD, INS | 121 | |
| (NHEt3)2(NH2Me2)[V12As8O40(HCO2)]·2H2O, Na5[V12As8O40(HCO2)]·18H2O | Deep green, deep blue | NaVO3·2H2O, As2O3, N2H4·HCl, N,N-dimethylformamide | EA, IR, UV-vis, TGA, magnetometry, single-crystal XRD | 119 | |||
| Na4[V12As8O40(H2O)]·23H2O | Dark blue | NaVO3, As2O3, H2O, N2H4·H2SO4, HCl | 4 °C, 2–3 d, pH = 6.0 | 53% based on V | IR, magnetometry, powder XRD, single-crystal XRD, INS | 121 | |
| Na4[V12As8O40(D2O)]·16.5D2O | NaVO3, As2O3, D2O, N2H4·H2SO4, DCl | 4 °C, 2–3 d, pH = 6.0 | IR, magnetometry, powder XRD, single-crystal XRD, INS | 121 | |||
| K3[H12V12O36(AsO)2(AsO4)]·12H2O | Dark green | NaVO3, As2O3, H2O, H2NC(CH2OH)3, KSCN, H2SO4 | 70 °C, 22 h, pH = 4.6 | 25% based on V | EA, IR, TGA, single-crystal XRD | 116 | |
| K6[H3KV12As3O39(AsO4)]·8H2O | Black grey | Zig-zag chain | KVO3, H2O, As2O5·5H2O, KSCN, H2SO4 | 1 h, 90 °C, pH = 3 | EA, IR, EPR, magnetometry, single-crystal XRD | 115 | |
| (NEt4)2[V12O12(OH)2(H2O)4(O3AsPh)10(HO3AsPh)4]·6H2O | Light green | (NnBu4)3[H3V10O28], PhAsO3H2, Et4NCl, MeOH/H2O | 100 °C, 17 h → 120 °C, 3 d | 30% based on V | EA, IR, TGA, single-crystal XRD | 162 | |
| (H7O3)2(NnBu4)2[(MeOH)2V12O14(OH)4(O3AsPh)10]·H2O | Light green | (NnBu4)3[H3V10O28], PhAsO3H2, MeOH/H2O | 100 °C, 17 h → 120 °C, 3 d | 20% based on V | EA, IR, TGA, single-crystal XRD | 162 | |
| Na4(H2O)10[{V12O14(OH)4(H2O)3(O3AsC6H4-4-NH2)10}]·1.5DMF·1.25H2O | Blue | NaVO3, NaN3, p-arsanilic acid, H2O, DMF, N2H4·H2O, HCl | 70 °C, pH = 4.9 | 43% | EA, IR, UV-vis, single-crystal XRD | 166 | |
| [Zn(en)2]2[Zn2(bpe)2V12As8O40(H2O)] | Brown | 1D straight chain | NH4VO3, As2O3, ZnCl2·7H2O, bpe, en, HNO3, H2O | 170 °C, 7 d | 59% based on V | EA, FT-IR, TGA, CV, single-crystal XRD | 125 |
| {[Zn(dien)]2(dien)2[Zn2V12As8O40(0.5H2O)]}2·6H2O | Cluster dimers | V2O5, As2O3, H2O, Zn(OAc)2·4H2O, dien | 160 °C, 3 d | 36% based on V2O5 | EA, IR, single-crystal XRD | 124 | |
| [{Zn(enMe)2}2(enMe)2{Zn2V12As8O40(H2O)}]·4H2O | Brown | V2O5, As2O3, enMe, Zn(OAc)2·4H2O, H2O | 180 °C, 7 d | 76% based on V | EA, IR, TGA, EPR, magnetometry, single-crystal XRD | 123 | |
| [Zn(enMe)2]2[(4,4′-bipy)Zn2V12As8O40(H2O)] | Brown | 1D straight chain | NH4VO3, As2O3, ZnCl2·7H2O, 4,4′-bipy, enMe, HNO3, H2O | 170 °C, 5 d | 60% based on V | EA, FT-IR, TGA, CV, magnetometry, single-crystal XRD | 126 |
| [Zn(en)2(H2O)][Zn(en)2(4,4′-bipy)Zn2V12As8O40(H2O)]·3H2O | Brown | 1D sinuate chain | NH4VO3, As2O3, ZnCl2·7H2O, 4,4′-bipy, en, HNO3, H2O | 170 °C, 5 d | 30% based on V | EA, FT-IR, TGA, CV, single-crystal XRD | 126 |
| [{Zn(en)3}2{Zn2V12As8O40(H2O)}]·4H2O·0.25bipy | Brown | Eight-shaped chiral helix | NH4VO3, As2O3, ZnCl2·7H2O, 4,4′-bipy, en, HNO3, H2O | 170 °C, 5 d | 39% based on V | EA, FT-IR, TGA, CV, single-crystal XRD | 126 |
| [Zn2(en)5][{Zn(en)2}{(bpe)HZn2V12As8O40(H2O)}2]·7H2O | Brown | 2D network | NH4VO3, As2O3, ZnCl2·7H2O, bpe, en, HNO3, H2O | 170 °C, 5 d | 37% based on V | EA, FT-IR, TGA, CV, UV-Vis, magnetometry, single-crystal XRD | 126 |
| [Cd(en)2]2[Cd2(en)2V12As8O40] | Brown | 1D chain | V2O5, As2O3, en, H2O, Cd(OAc)2·2H2O | 180 °C, 6 d | 52% based on V | EA, IR, TGA, EPR, magnetometry, powder XRD, single-crystal XRD | 127 |
| [Cd(enMe)3]2[(enMe)2Cd2V12As8O40(0.5H2O)]·5.5H2O | Brown | Layer | V2O5, As2O3, H2O, enMe, Cd(OAc)2·2H2O | 170 °C, 3 d | 42% based on V | EA, IR, TGA, UV-Vis, magnetometry, powder XRD, single-crystal XRD | 128 |
| [Cd(enMe)2]2[Cd2(enMe)2V12As8O40(0.5H2O)] | Brown | 1D linear chain | V2O5, As2O3, H2O, enMe (excess), Cd(OAc)2·2H2O | 170 °C, 3 d | 32% based on V | EA, IR, TGA, UV-Vis, magnetometry, powder XRD, single-crystal XRD | 128 |
| V13 | |||||||
| [Zn(2,2′-bipy)3]4[(ppz){{Zn(tepa)}2ZnV13As8O41(H2O)}2][V14As8O42(0.5H2O)]2·4H2O | Brown | 3D network | V2O5, As2O3, H2O, dien, Zn(OAc)2·4H2O, 2,2′-bipy | 160 °C, 3 d, pH = 7.45 → 8.35 | 39% based on V2O5 | EA, IR, TGA, magnetometry, powder XRD, single-crystal XRD | 124 |
| [Cd(en)3][Cd(phen)(en)(H2O)2][Cd(en)V13As8O41(H2O)]·1.5H2O | Brown | NH4VO3, As2O3, 1,10-phen, en, CdCl2·7H2O, HNO3, H2O | 170 °C, 7 d | 46% based on V | EA, FT-IR, TGA, CV, powder XRD, single-crystal XRD | 125 | |
| [Cd(phen)2(en)]2[Cd(phen)V13As8O41(H2O)]·21H2O·phen | Brown | NH4VO3, As2O3, 1,10-phen, en, CdCl2·7H2O, HNO3, H2O | 170 °C, 7 d | 55% based on V | EA, FT-IR, TGA, CV, magnetometry, powder XRD, single-crystal XRD | 125 | |
| [Cd(dien)2]2[Cd(dien)V13As8O41(H2O)]·4H2O | Brown | V2O5, As2O3, dien, H2O Cd(OAc)2·2H2O | 180 °C, 6 d | 61% based on V | EA, IR, EPR, TGA, magnetometry, single-crystal XRD | 127 | |
| {[V13As8NiClO41][Ni(en)2(H2O)][Ni(en)2]}{[Ni(en)2(H2O)2]0.5}·4H2O | Black | 1D chain | V2O5, As2O3, H2C2O4·2H2O, en, NiCl2·6H2O, H2O | 160 °C, 3 d | 68% based on V | EA, IR, TGA, magnetometry, single-crystal XRD | 129 |
| V14 | |||||||
| (NH4)6[V14As8O42(SO3)] | Brown | NH4VO3, As2O3, H2O, Na2S2O4, NH3, NH4SCN | 80 °C, 1–2 d (not stirred) | EA, IR, UV-vis, single-crystal XRD | 130 | ||
| (NH4)6[V14As8O42(SO4)] | Brown | NH4VO3, As2O3, H2O, N2H6SO4, NH3, NH4SCN | 90 °C, 3 d (not stirred) | EA, IR, UV-vis, single-crystal XRD | 130 | ||
| (NMe4)4[V14As8O42(H2O)] | Dark green | NaVO3, As2O3, NMe4Cl, H2O, N2H5Cl | 70 °C, 7 d (not stirred) | EA, IR, UV-vis, single-crystal XRD | 130 | ||
| (NMe4)4[V14As8O42(H2O)0.5] | Dark brown | V2O5, As2O3, Me4NOH, H2O | 200 °C, 2 d | 89% based on V | EA, IR, single-crystal XRD | 131 | |
| (NH4)2(NMe4)4[V14As8O42(SO4)] | Black | NH4VO3, As2O3, NiSO4·6H2O, NMe4OH, H2O | 160 °C, 5 d | 54% based on V | EA, IR, TGA, single-crystal XRD | 134 | |
| (NEt4)3[H6V12AsO40(VO)2]·20H2O | Dark red | NH4VO3, Na2HAsO4, NH2OH, HCl, H2O, Et4NBr | Reflux temperature (1.5 h) → r.t. | EA, single-crystal XRD | 117 | ||
| (H2en)3.5[V14As8O42(PO4)]·2H2O | Black | V2O5, As2O3, H3PO4, en, H2C2O4·2H2O, H2O | 160 °C, 3 d | 80% based on V | EA, IR, TGA, XPS, ESR, single-crystal XRD | 135 | |
| (H2enMe)2[V14As8O42(H2O)]·3H2O | Black | Triple clusters | NH4VO3, As2O3, FeSO4·4H2O, H3PO4, enMe, H2C2O4·2H2O, H2O, NH3·H2O | 160 °C, 3 d | 80% based on V | EA, IR, UV-vis, XPS, EPR, powder XRD, single-crystal XRD, magnetometry | 136 |
| (Hen)2(H2en)[V14As8O42(H2O)]·2.33H2O | Black | V2O5, As2O3, CuCl2·2H2O, en, H2O, HCl | 170 °C, 7 d, pH = 6.0 | 64% based on V | EA, IR, TGA, single-crystal XRD | 140 | |
| [NH2(CH2)4NH2]4[V14As8O42(SO4)]·(HSO4)2 | Black | Layer | VOSO4·H2O, As2O5, ppz, H2O | 180 °C, 6 d | EA, IR, TGA, single-crystal XRD | 132 | |
| [NH2(CH2CH2)2NH2]3[V14As8O42(SO4)]·6.5H2O | Brown | VOSO4·H2O, As2O5, HN(CH2CH2)2NH, H2O | 180 °C, 6 d | 76% based on V | EA, IR, TGA, single-crystal XRD, magnetometry | 133 | |
| H6[Cl2V14O16(OH)8(O3AsC6H4-4-NH2)10]·8DMF·16H2O | Turquoise | NaVO3, (p-aminophenyl)arsonic acid, H2O, DMF, HCl, N2H4·H2O | 70 °C, 30 min, pH = 4.5 | 19% | EA, IR, ESI-MS, UV-vis, single-crystal XRD | 165 | |
| K7[V14AsO40]·12H2O | Dark blue | KVO3, As2O5·5/3H2O, KSCN, H2O, H2SO4, KOH | 70–75 °C, 16 h, pH = 4.6 | EA, IR, Raman, TGA, UV-vis, EPR, single-crystal XRD, magnetometry | 106 | ||
| Rb5[V14As8O42(Cl)]·2H2O | Brown | V2O5, V2O3, V (mesh), H5As3O10, RbOH, H2O | 200 °C, 2.5 d | 40% based on V | EA, IR, UV-vis, single-crystal XRD | 116 | |
| [Zn(bbi)2]2[V14As8O42(H2O)] | Dark green | 2D network | NH4VO3, bbi, ZnCl2·2H2O, NaOAc, H2C2O4·2H2O, tris(hydroxymethyl)amino-methane, Na3AsO4·H2O, H2O, HCl | 88% based on V | EA, IR, powder XRD, magnetometry | 148 | |
| [Zn(2,2′-bipy)2]2[V14As8O42(H2O)]·H2O | Brown | 1D tubular chain | V2O5, As2O5, Zn(OAc)2·2H2O, 2,2′-bipy, NMe4OH, H2O | 160 °C, 6 d | 33% based on V | EA, IR, EPR, single-crystal XRD, magnetometry | 144 |
| [Zn(2,2′-bipy)3]2[V14As8O42(H2O)]·4H2O | Brown | V2O5, As2O3, Zn(OAc)2·2H2O, 2,2′-bipy, ppz, H2O | 180 °C, 6 d | 73% based on V | EA, IR, EPR, TGA, powder XRD, single-crystal XRD, magnetometry | 138 | |
| [{(H2O)Zn(4,4′-bipy)}2{V14As8O42(H2O)}]·2H2O | Black | 2D network | V2O5, As2O3, Zn(OAc)2·2H2O, 4,4′-bipy, H2O | 160 °C, 7 d | 23% based on V | EA, IR, XPS, TGA, CV, single-crystal XRD | 143 |
| [Zn(2,2′-bipy)(dien)]2[V14As8O42(H2O)]·2H2O | Brown | V2O5, As2O3, Zn(OAc)2·2H2O, 2,2-bipy, dien, H2O | 180 °C, 6 d | 68% based on V | EA, IR, EPR, TGA, powder XRD, single-crystal XRD, magnetometry | 138 | |
| [{(H2O)Zn(1,10-phen)2}2{V14As8O42(H2O)}]·4H2O | Black | 2D supermolecular array | V2O5, As2O3, Zn(OAc)2·2H2O, 1,10-phen, H2O | 160 °C, 7 d | 39% based on V | EA, IR, XPS, TGA, CV, single-crystal XRD | 143 |
| [Zn(phen)3]2[V14As8O42(H2O)]·4H2O | Brown | Layer-like arrangement | V2O5, As2O3, Zn(OAc)2·6H2O, phen, dien, H2O | 180 °C, 3 d | 62% based on V | EA, IR, TGA, single-crystal XRD, fluorescence | 141 |
| [Zn(en)2][(H2O)Zn(en)2V14As8O42(H2O)]·H2O | Black | Infinite 1D channels | NH4VO3, As2O3, Zn(NO3)2·6H2O, HNO3, en, H2O | 160 °C, 7 d | 45% based on V | EA, IR, TGA, CV, single-crystal XRD | 143 |
| [{(H2O)Zn(2,2′-bipy)2}{Zn(2,2′-bipy)2}V14As8O42(H2O)0.5]2[{(H2O)Zn(2,2′-bipy)2V14As8O42(H2O)0.5}2{Zn(2,2′-bipy)2}2]·3H2O | Black | Cluster dimers | V2O5, As2O3, Zn(OAc)2·2H2O, 2,2′-bipy, H2O | 160 °C, 7 d | 29% based on V | EA, IR, XPS, TGA, CV, single-crystal XRD | 143 |
| [Cd(phen)3]2[V14As8O42(H2O)]·2H2O | Brown | Layer-like arrangement | V2O5, As2O3, Cd(OAc)2·6H2O, phen, dien, H2O | 150 °C, 5 d | 63% based on V | EA, IR, TGA, single-crystal XRD, fluorescence | 141 |
| [Cd(1,10-phen)3]2[V14As8O42(H2O)0.5]·0.5H2O | Black | NaVO3·2H2O, As2O3, Cd(OAc)2, 1,10-phen, NaOAc/HOAc | 175 °C, 5 d | 50% based on V | EA, IR, EPR, TGA, CV single-crystal XRD | 140 | |
| [Cd(2,2′-bipy)3][Cd(dien)V14As8O42(H2O)] | Brown | 1D wave-like chain | V2O5, As2O3, CdSO4·8H2O, 2,2′-bipy, H2C2O4·2H2O, dien, H2O | 160 °C, 3 d | 51% based on V | EA, IR, EPR, single-crystal XRD, magnetometry | 144 |
| [Cu(en)2]2[V14As8O42(H2O)]·2.5H2O | Brown | 1D chain | NH4VO3, As2O3, CuCl2·2H2O, en, HNO3, H2O | 160 °C, 7 d | 33% based on V | EA, IR, TGA, single-crystal XRD | 140 |
| [Cu(en)2]3[V14As8O42(CO3)]·10H2O | Black | 2D layered network | V2O5, As2O3, CuCl2·2H2O, en, H2C2O4·2H2O, H2O | 160 °C, 3 d | 80% based on V | EA, IR, TGA, single-crystal XRD | 149 |
| [Cu(bbi)]4[V14As8O42(H2O)] | Green | 3D network | NH4VO3, bbi, Cu(OAc)2·3H2O, NaOAc, H2C2O4·2H2O, tris(hydroxymethyl)amino-methane, Na3AsO4·H2O, H2O, HCl | 170 °C, 5 d | 62% based on V | EA, IR, single-crystal XRD | 148 |
| [Ni(bbi)2]2[V14As8O42(H2O)] | Dark green | 2D network | NH4VO3, bbi, Ni(NO3)2·6H2O, NaOAc, H2C2O4·2H2O, tris(hydroxymethyl)aminomethane, Na3AsO4·H2O, H2O, HCl | 170 °C, 5 d | 82% based on V | EA, IR, powder XRD, single-crystal XRD, magnetometry | 148 |
| [Ni(en)2]3[V14As8O42(SO4)]·4.5H2O | Black | 2D sinusoidal layer | V2O5, As2O3, en, H2C2O4·2H2O, H2O, NiSO4·6H2O | 160 °C, 3 d | 73% based on V | EA, IR, EPR, XPS, single-crystal XRD, magnetometry | 145 |
| [Ni(en)2]3[V14As8O42(HPO3)]·4H2O | Brown | 2D puckery layer | V2O5, As2O3, H3PO3, en, Ni, Ni(OAc)2·4H2O, H2O | 160 °C, 4 d | 16% based on V | EA, IR, EPR, single-crystal XRD | 144 |
| (2,2′-bipy)[Ni(2,2′-bipy)3]2[V14As8O42(H2O)]·3H2O | Brown | V2O5, As2O3, H2O, Ni(OAc)2·4H2O, en, 2,2′-bipy | 160 °C, 3 d | 46% based on V | EA, IR, EPR, TGA, UV-vis, XPS, single-crystal XRD | 139 | |
| [Ni(enMe)3]4[Ni(enMe)2][V14As8O42(NO3)]2·8H2O | Black | Cluster dimers | NH4VO3, As2O3, Ni(NO3)2·6H2O, bpp, enMe, HNO3, H2O | 170 °C, 7 d | 45% based on V | EA, IR, TGA, CV, powder XRD, single-crystal XRD, magnetometry | 125 |
| [{Ni(en)2}4(4,4′-bipy)4{Ni(H2O)2}]2[V14As8O42(NO3)]4·16H2O | Black | Box-like framework | NH4VO3, As2O3, Ni(NO3)2·6H2O, 4,4′-bipy, HNO3, en, H2O | 170 °C, 5 d | 70% based on V | EA, IR, TGA, CV, single-crystal XRD, magnetometry | 146 |
| [Ni(en)2(H2O)2]2[{Ni(en)2(H2O)}2V14As8O42(NO3)][{Ni(en)2}V14As8O42(NO3)]·6H2O | Black | 1D chain | NH4VO3, As2O3, Ni(NO3)2·6H2O, 1,3-bis(4-pyridyl)propane, HNO3, en, H2O | 170 °C, 5 d | 10% based on V | EA, IR, single-crystal XRD | 146 |
| [Co(2,2′-bipy)2]2[V14As8O42(H2O)]·H2O | Black | 1D tubular chain | VOSO4, As2O3, CoC2O4·2H2O, 2,2′-bipy, H2O, dien | 160 °C, 9 d, pH = 5.5 | 30% based on V | EA, IR, TGA, single-crystal XRD, magnetometry | 147 |
| [Co(2,2′-bipy)3]2[V14As8O42(H2O)]·3H2O | Brown | V2O5, As2O3, H3PO3, H2O, H2C2O4·2H2O, Co(OAc)2·4H2O, en, 2,2′-bipy | 160 °C, 3 d | 52% based on V | EA, IR, TGA, UV-vis, XPS, EPR, single-crystal XRD | 139 | |
| [Co(dien)2]2[V14As8O42(H2O)]·3.5H2O | Brown | Soft channels | V2O5, Na3AsO4, CoCl2·6H2O, dien, H2O, H2SO4 | 170 °C, 7 d, pH = 8.0 | 33% based on V | EA, IR, TGA, single-crystal XRD | 140 |
| [Co(bbi)2]2[V14As8O42(H2O)] | Dark green | 2D network | V2O5, bbi, Co(OAc)2·4H2O, H2C2O4·2H2O, NaOAc, tris(hydroxymethyl)aminomethane, Na3AsO4·H2O, H2O, HCl | 170 °C, 5 d | 55% based on V | EA, IR, powder XRD, single-crystal XRD, magnetometry | 148 |
| [Co(en)3][Co(en)2V14As8O42(H2O)]·16H2O | Black | 1D chain | NH4VO3, As2O3, Co(NO3)2·6H2O, 4,4′-bipy, en, HNO3, H2O | 170 °C, 7 d | 40% based on V | EA, IR, TGA, CV, powder XRD, single-crystal XRD | 125 |
| [Mn(1,10-phen)3]2[V14As8O42(H2O)0.5]·0.5H2O | Green | NH4VO3, As2O3, KMnO4, 1,10-phen, H2O | 160 °C, 7 d | 10% based on V | EA, IR, EPR, TGA, single-crystal XRD | 140 | |
| [{La(H2O)6}2V14As8O42(SO3)]·8H2O | Brown | 2D layered network | (NH4)6[V14As8O42(SO3)], La(NO3)3·6H2O, H2O | 7 d | 32% based on (NH4)6[V14As8O42(SO3)] | EA, IR, EPR, TGA, diffuse reflectance, powder XRD, single-crystal XRD | 150 |
| [{Ce(H2O)6}2V14As8O42(SO3)]·8H2O | Brown | 2D layered network | (NH4)6[V14As8O42(SO3)], Ce(NO3)3·6H2O, H2O | 7 d | 32% based on (NH4)6[V14As8O42(SO3)] | EA, IR, EPR, TGA, diffuse reflectance, powder XRD, single-crystal XRD | 150 |
| [{Sm(H2O)6}2V14As8O42(SO3)]·8H2O | Brown | 2D layered network | (NH4)6[V14As8O42(SO3)], Sm(NO3)3·6H2O, H2O | 7 d | 27% based on (NH4)6[V14As8O42(SO3)] | EA, IR, EPR, TGA, diffuse reflectance, powder XRD, single-crystal XRD | 150 |
| V15 | |||||||
| K6[V15As6O42(H2O)]·8H2O | Brown | 3D network | KVO3, As2O3, KSCN, KOH, H2O, N2H4·H2SO4 | 85 → 20 °C, pH = 8.4 | 55% | EA, IR, TGA, single-crystal XRD, magnetometry | 151 |
| [Zn(H2O)4]2[H2V15As6O42(H2O)]·2H2O | Black | V2O5, As2O3, H2C2O4·2H2O, en, Zn(OAc)2·2H2O, H2O | 160 °C, 3 d | 53% based on As | EA, IR, ESR, single-crystal XRD, magnetometry, third-order NLO | 153 | |
| [Zn(en)2][Zn(en)2(H2O)2][{Zn(en)(enMe)}V15As6O42(H2O)]·4H2O | Black | V2O5, As2O3, en, enMe, Zn(OAc)2·2H2O, H2O | 160 °C, 6 d, pH = 8.5 | 52% based on V | EA, IR, TGA, single-crystal XRD | 154 | |
| [Zn2(enMe)2(en)3][{Zn(enMe)2}V15As6O42(H2O)]·4H2O | Black | V2O5, As2O3, en, enMe, Zn(OAc)2·2H2O, H2O | 160 °C, 6 d | 37% based on V | EA, IR, TGA, single-crystal XRD | 154 | |
| (Hen)2[{{Zn(en)2}2V15As6O42(H2O)}2{Zn(en)2}]·3H2O | Black | Cluster dimers | VOSO4, As2O3, ZnO, en, HCl, H2O | 160 °C, 4 d, pH = 7 | 58% based on V | EA, IR, TGA, single-crystal XRD | 155 |
| [Zn2(dien)3(H2O)2]0.5[{Zn2(dien)3}V15As6O42(H2O)]·2H2O | Brown | 1D helical chain | V2O5, As2O3, dien, Zn(OAc)2·2H2O, H2O | 160 °C, 3 d | 57% based on V | EA, IR, TGA, EPR, single-crystal XRD | 156 |
| [Cu(en)2]1.5[H3V15As6O42(H2O)]·3H2O | Black | 1D sinusoidal chain | V2O5, As2O3, H2C2O4·2H2O, CuSO4·5H2O, en, H2O | 160 °C, 3 d | 67% based on V | EA, IR, single-crystal XRD | 158 |
| [Cu(enMe)2]2.5[HV15As6O42(H2O)]·2H2O | Black | Brick-wall-like 2D layer | V2O5, As2O3, H2SO4, enMe, H2C2O4·2H2O, CuSO4·5H2O, H2O, NH3·H2O | 160 °C, 3 d, pH = 10 | 51% based on V | EA, IR, TGA, UV-vis, EPR, XPS, magnetometry, single-crystal XRD | 136 |
| [Ni(2,2′-bipy)3]2[{Ni(en)2}V15As6O42(H2O)]·9.5H2O | Brown | 1D straight chain | V2O5, As2O3, 2,2′-bipy, Ni(OAc)2·4H2O, en, H2O | 160 °C, 3 d | 65% based on V | EA, IR, TGA, EPR, single-crystal XRD | 156 |
| [Co(en)3][{Co(en)2}2V15As6O42]·4H2O | Black | 1D chain | V2O5, As2O3, H3PO3, Co(OAc)2, en, H2O | 160 °C, 3 d | 78% | IR, single-crystal XRD, magnetometry | 157 |
| [Co(enMe)2]3[V15As6O42(H2O)]·2H2O | Brown | 2D framework | V2O5, As2O3, enMe, H2C2O4·2H2O, CoSO4·4H2O, H2O | 160 °C, 6 d | 27% based on V | EA, IR, TGA, EPR, single-crystal XRD | 156 |
| V16 | |||||||
| (HNEt3)2[{Br2(H2O)4}(VO)16(OH)8(O4AsPh)2(O3AsPh)8]·6MeCN | Blue | VBr3, PhAsO3H2, NEt3, MeCN | 80 °C, 30 min | 62% based on V | EA, IR, ESI-MS, powder XRD, single-crystal XRD | 163 | |
| (HNEt3)2[{Cl2(H2O)4}(VO)16(OH)8(O4AsPh)2(O3AsPh)8]·2H2O | Blue | VCl3, PhAsO3H2, NEt3, MeCN | 80 °C, 30 min | 65% based on V | EA, IR, ESI-MS, powder XRD, single-crystal XRD | 163 | |
| H5[{Cl4(H2O)2}(VO)16O16(O3AsPh)8]Cl·4H2O·3MeCN | Green | VCl3, Dy(NO3)3·xH2O, PhAsO3H2, NEt3, MeCN | 80 °C, 30 min | 12% based on V | EA, IR, ESI-MS, powder XRD, single-crystal XRD | 163 | |
| [Zn2(dien)3][{Zn(dien)}2V16As4O42(H2O)]·3H2O | Brown | 1D linear chain | V2O5, As2O3, H2O, dien, Zn(OAc)2·4H2O | 170 °C, 4 d pH = 7.15 → 8.05 | 31% based on V2O5 | EA, IR, TGA, magnetometry, single-crystal XRD | 159 |
| V20 | |||||||
| [{Cl4(H2O)2}(VO)20O16(OH)4(O3AsPh)8]·7H2O·3MeCN | Green | VCl3, Dy(NO3)3·xH2O, PhAsO3H2, NEt3, MeCN | 80 °C, 30 min | 58% based on V | EA, IR, ESI-MS, powder XRD, single-crystal XRD | 163 | |
| V24 | |||||||
| H10[{Cl6}(VO)24O24(O3AsPh)8]Cl4·10H2O·2MeCN | Green | VCl3, Dy(NO3)3·xH2O, PhAsO3H2, NEt3, MeCN | 80 °C, 30 min | 52% based on V | EA, IR, ESI-MS, powder XRD, single-crystal XRD | 163 | |
| Formula | Colour | Characteristics of crystal structurea | Reactants | Reaction conditions | Yield | Characterised via | Ref. |
|---|---|---|---|---|---|---|---|
| a Dimensionality resulting from hydrogen bonding networks is not considered. | |||||||
| V14 | |||||||
| (NH4)4[V14Sb8O42]·2H2O | Green brown | Layer-like arrangement | NH4VO3, Sb2O3, theed, H2O | 150 °C, 14 d | 40% based on Sb | Powder XRD, single-crystal XRD | 185 |
| (H2en)2[V14Sb8O42(H2O)]·3H2O | Black | Layer-like arrangement | NH4VO3, Sb2O3, N,N,N′,N′-tetramethylethylenediamine, H2O | 180 °C, 7 d | 37% based on Sb | EA, IR, single-crystal XRD | 193 |
| (H2ppz)2[V14Sb8O42(H2O)] | Black | Layer-like arrangement | NH4VO3, Sb2O3, 1-methylpiperazine, H2O | 180 °C, 7 d | Powder XRD, SEM, single-crystal XRD | 193 | |
| [(H2en)2{V14Sb8O42(H2O)}]·(en)·4H2O | Black | 1D double chain | VOSO4, Sb2O3, H2O, C2N2H8 (en) | pH = 7.5, 175 °C, 4 d | 61% based on Sb | EA, ICP, IR, single-crystal XRD | 184 |
| [V14Sb8(Haep)4O42(H2O)]·4H2O | Brown green | Chain | NH4VO3, Sb2O3, aep, H2O | 180 °C, 7 d | 24% based on Sb | EA, UV-vis, DTA-TGA, single-crystal XRD, magnetometry | 188 |
| [Zn(dien)2]2[{Zn(dien)}2(V14Sb8O42)2(H2O)]·4H2O | Brown | 1D zig-zag chain | Sb2O3, V2O5, Zn(OAc)2·2H2O, dien, H2O, NaOH | pH = 9.5, 180 °C, 7 d | 25% based on V | EA, IR, XPS, TGA, single-crystal XRD | 189 |
| [{Co(en)2}2V14Sb8O42(H2O)]·6H2O | Black | 2D network | V2O5, Sb2O3, H2C2O4·2H2O, CoC2O4·2H2O, H2O, en | pH = 9.0, 160 °C, 9 d | 46% based on Sb | EA, ICP, IR, single-crystal XRD | 191 |
| V15 | |||||||
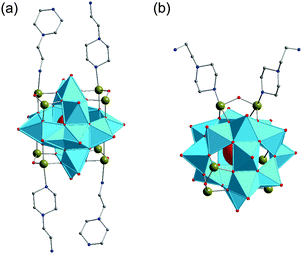
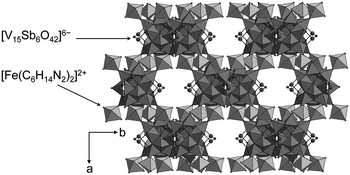
| (H3tren)2[V15Sb6O42]·0.33(tren)·nH2O (n = 3–5) | Brown greenish | Hexagonal layer | NH4VO3, Sb2O3, tren, H2O | 150 °C, 7 d | 70% based on Sb | EA, IR, UV-vis, Raman, DTA-TGA-MS, single-crystal XRD, magnetometry | 186 |
| (H2aep)2[V15Sb6(Haep)2O42(H2O)]·2.5H2O | Black | Rows | NH4VO3, Sb2O3, aep, H2O | 160 °C, 7 d | 64% based on Sb | EA, UV-vis, DTA-TGA, single-crystal XRD, magnetometry | 188 |
| [Ni(dien)2]3[V15Sb6O42(H2O)]·nH2O (n = 8, 12) | Brown | Layer-like arrangement | NH4VO3, Sb2O3, NiCl2·6H2O, dien, H2O | 130 °C/150 °C, 7 d | 36% based on Sb | EA, IR, DTA-TGA, single-crystal XRD, SEM | 196 |
| [Ni(aepda)2]2[{Ni(aepda)2}V15Sb6O42(H2O)]·8H2O | Brown | Double clusters | NH4VO3, Sb2O3, NiCl2·6H2O, aepda, H2O | 150 °C, 7 d | 60% based on Sb | EA, IR, DTA-TGA, powder XRD, single-crystal XRD, SEM, magnetometry | 194 |
| [Ni(Htren)2][Ni2(tren)3(V15Sb6O42(H2O)0.5)]2·H2O | Brown | 1D double chain | NH4VO3, Sb2O3, NiCl2·6H2O, tren, H2O | 150 °C, 5 d | EA, IR, UV-vis, powder XRD, single-crystal XRD | 195 | |
| [Co(aepda)2]2[{Co(aepda)2}V15Sb6O42(H2O)]·5H2O | Brown | Double clusters | NH4VO3, Sb2O3, CoCl2·6H2O, aepda, H2O | 130 °C, 7 d | 45% based on Sb | EA, IR, DTA-TGA, single-crystal XRD, SEM, magnetometry | 194 |
| [Co(tren)(H2O)]3[V15Sb6O42(H2O)]·H2O | Dark brown | 2D network | NH4VO3, Sb2O3, CoCl2·6H2O, tren (conc. 50%) | 170 °C, 7 d | 60% based on Sb | EA, IR, EDXS, DTA-TG, powder XRD, single-crystal XRD | 192 |
| [Co2(tren)3]2[Co(tren)(en)][{V15Sb6O42(H2O)(Co(tren)2)}V15Sb6O42(H2O)]·nH2O (n ≈ 11) | Brown | 1D double chain | NH4VO3, Sb2O3, CoCl2·6H2O, tren (conc. 75%) | 170 °C, 7 d | 35% based on Sb | EA, IR, EDXS, DTA-TG, powder XRD, single-crystal XRD | 192 |
| [{Fe(dach)2}3{V15Sb6O42(H2O)}]·8H2O | Dark red | Porous 3D network | NH4VO3, Sb2O3, FeSO4·7H2O, dach, H2O | 160 °C, 7 d | 23% based on V | EA, IR, UV-vis, single-crystal XRD, magnetometry | 197 |
| V16 | |||||||
| (H2aep)4[V16Sb4O42]·2H2O | Brown | Chain | NH4VO3, Sb2O3, aep, H2O | 150 °C, 7 d | Single-crystal XRD | 185 | |
| [Zn2(dien)3][{Zn(dien)}2V16Sb4O42(H2O)]·4H2O | Brown red | 1D linear chain | Sb2O3, V2O5, Zn(OAc)2·2H2O, dien, H2O, NaOH | pH = 7.8–8.3, 180 °C, 7 d | 75% based on V | EA, IR, XPS, TGA, single-crystal XRD | 189 |
| [Zn(tren)(H2tren)]2[V16Sb4O42(H2O)]·nH2O (n = 6–10) | Brown | Layer-like arrangement | NH4VO3, Sb2O3, Zn(NO3)2·6H2O, tren, H2O | pH = 12.5, 130 °C, 7 d | 47% based on Sb | EA, IR, DTA-TGA, powder XRD, single-crystal XRD, SEM, magnetometry | 190 |
| [Ni(dien)2]4[V16Sb4O42(H2O)] | Black | Layer-like arrangement | NH4VO3, Sb2VO5, NiCl2·6H2O, dien, H2O | 150 °C, 7 d | Powder XRD, single-crystal XRD, SEM | 196 | |
| [Co(tren)(H2tren)]2[V16Sb4O42(H2O)]·6H2O | Brown | Layer-like arrangement | NH4VO3, Sb2O3, CoCl2·6H2O, tren (conc. 25%) | 130 °C, 7 d | 30% based on Sb | EA, IR, EDXS, DTA-TG, powder XRD, single-crystal XRD | 192 |
| V20 | |||||||
| [V16Sb4O42(H2O){VO(dach)2}4]·(dach)·10H2O | Dark brown | Porous 3D network | Sb2O3, NH4VO3, dach, H2O | 150 °C, 7 d | 60% based on V | EA, IR, EDXS, UV-vis, single-crystal XRD, magnetometry | 187 |
As vanadium precursors for the synthesis of the AsPOVs (Table 3) and SbPOVs (Table 4), V2O5 and NH4VO3 were extensively used, whilst NaVO3, VOSO4 and vanadium halides were employed less frequently. The POV structures decorated with handle-like {E2O5} groups (E = As, Sb) are typically viewed as being derived from the {V18O42} archetype, with only some exceptions which are discussed in Section 3. In contrast to the SiIV and GeIV atoms displaying tetrahedral {EO4} geometries in the heteroPOV structures, the heavier group 15 elements, As and Sb, usually adopt trigonal pyramidal {EO3} geometries and show the formal oxidation state +3; however, very rarely tetrahedral {AsO4}3− units and arsenic atoms in the formal oxidation state +5 were also identified. The As–O bonds (dAs–O = 1.52–2.02 Å) are usually longer than the terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (dV
O bonds (dV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O = 1.52–1.68 Å), but shorter than the bridging V–O bonds (dV–O = 1.72–2.33 Å). The Sb–O bonds (dSb–O = 1.90–2.04 Å) fall in the range of V–O bond lengths, but these are remarkably longer than the V
O = 1.52–1.68 Å), but shorter than the bridging V–O bonds (dV–O = 1.72–2.33 Å). The Sb–O bonds (dSb–O = 1.90–2.04 Å) fall in the range of V–O bond lengths, but these are remarkably longer than the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.
O bonds.
3.2 Polyoxovanadatoarsenates (AsPOVs)
AsPOVs with the encapsulated AsO4 units. In the early 1990s Müller, Gatteschi and colleagues described the synthesis and magnetic properties of the compound K6[H3KV12As3O39(AsO4)]·8H2O exhibiting electron delocalisation effects.115 The central constituent of this compound obtained upon reduction of an aqueous solution of potassium metavanadate KVO3 is the mixed-valent [H3KVIV4VV8AsV3O39(AsVO4)]6− polyoxoanion with approximate C3 symmetry (Table 3). The structure of this AsPOV consists of nine {VO6} octahedra, three {VO4} tetrahedra, and four {AsVO4} tetrahedra. One of these arsenate units {AsO4}3− is covalently enclosed within the AsPOV shell (Fig. 12). The terminal O atoms of three other, peripheral {AsVO4} groups are protonated. A potassium ion caps the monolacunary [H3V12As3O39(AsO4)]7− polyoxoanion. The electron population in this AsPOV was confirmed by bond valence sum analysis and manganometric titration. In the structure, K6[H3KV12As3O39(AsO4)]·8H2O displays zig-zag chains in which the neighbouring [H3KVIV4VV8AsV3O39(AsVO4)]6− polyoxoanions are linked through the K⋯Oterm–V interactions.
Another potassium-containing compound, K3[H12V12O36(AsO)2(AsO4)]·12H2O (Table 3), displays a structure that can be formally viewed as α-Keggin-type {VIV6VV6O36(AsVO4)} core with two of its six tetragonal {V4O4} faces additionally capped by two {AsVO} groups.116 As in the case of [H3KVIV4VV8AsV3O39(AsVO4)]6−, the tetrahedral {AsO4}3− unit is enclosed within the {V12O36} cage of the mixed-valent [H12VIV6VV6O36(AsO)2(AsO4)]3− polyoxoanion. The twelve O sites of this AsPOV are protonated and three K+ cations then compensate the −3 net charge of the polyoxoanion. In the crystal structure, each of these K+ cations is coordinated by four water molecules and is involved in bonding to four adjoined [H12V12O36(AsO)2(AsO4)]3− polyoxoanions through (weak) K–O bonds.
Another type of a bicapped Keggin structure with two capping {VVO} groups instead of two {AsVO} ones was found for the fully-oxidised [H6VV12O36(VVO)2(AsO4)]3− polyoxoanion isolated as (NEt4)3[H6V12AsO40(VO)2]·20H2O (Table 3).117 The α-Keggin core of this AsPOV consists of twelve {VO6} octahedra and the covalently enclosed, central {AsO4} tetrahedron whose O atoms are shared by four {V3O13} groups of edge-sharing octahedra.
Neutral AsPOV with the capping AsO4 units. The tetrahedral {AsO4}3− units capping tetragonal vanadium oxide faces were observed in hydrothermally synthesised [VIV8VV2AsV2O26(H2O)]·8H2O (Table 3).118 Its crystal structure shows an extended 3D network in which the neutral, mixed-valent POVs are joined through {AsO4} bridging groups. Notably, this compound exhibited catalytic activity for phenol hydroxylation, offering high selectivity to hydroquinone.
Discrete mixed-valent AsPOVs. The first polyoxoanions from this class of AsPOVs were presented by Müller and co-workers, in 1991.119 The formate-enclosing β-[HCO2@VIV6VV6As8O40]3− and β-[HCO2@VIV8VV4As8O40]5− components of the mixed-valent compounds (NHEt3)2(NH2Me2)[V12As8O40(HCO2)]·2H2O and Na5[V12As8O40(HCO2)]·18H2O (Table 3), respectively, display different electron populations and different types of spin–spin interactions, albeit their AsPOV shells with encapsulated formiate ions are isostructural and possess D4h symmetries. The strength of the spin–spin coupling in these polyoxoanions composed of four handle-like {As2O5} groups and twelve {VO5} square pyramids was shown to be influenced by the number of VIV centres: the larger number of these centres, the stronger coupling. Their magnetic properties were additionally studied by Gatteschi and colleagues.120 Although the [HCO2@VIV6VV6As8O40]3− and [HCO2@VIV8VV4As8O40]5− polyoxoanions are isonuclear, they exhibit dissimilar magnetic properties due to the different AsPOV spin topologies and delocalisation effects of the d1-VIV electrons. According to the magnetic susceptibility data, the AsPOV trianion is characterised by ferromagnetic coupling between delocalised and localised VIV ions and the AsPOV pentaanion features a singlet (S = 0) ground state because of strongly coupled, delocalised VIV ions. Furthermore, it was stated that field-dependent relaxation effects may influence the EPR spectra (at X-band) of these dodecanuclear species.
About 10 years later, Güdel and colleagues conducted an INS study for three new, but related dodecanuclear β-AsPOVs isolated as Na4[VIV8VV4As8O40(H2O)]·23H2O, the deuterated derivative Na4[VIV8VV4As8O40(D2O)]·16.5D2O, and (NHEt3)4[VIV8VV4As8O40(H2O)]·H2O (Table 3).121 The goal of the study was to gain insights into magnetic exchange interactions in these mixed-valent spin structures possessing two outer (dV⋯V = 3.410–3.450 Å) and one inner (or central) V4 squares (dV⋯V = 5.26–5.31 Å) bridged by [As2O5]4− groups. The magnetic transitions (up to four) between the S = 0 ground state of the {VIV8VV4As8} building block and its excited states were identified by INS. By this study, the authors confirmed “the essential correctness of an earlier model developed on the basis of magnetic and EPR measurements”120 that were performed for the aforementioned [HCO2@VIV8VV4As8O40]5− polyoxoanion.
The family of the {VIV8VV4As8}-type compounds119–121 was extended by the compound (NH4)4[V12As8O40(H2O)]·4H2O obtained under solvothermal conditions using the mixtures of ethyl ether/H2O or ethanol/H2O as reducing agents (Table 3).122 The building block of this compound is the mixed-valent β-[H2O@VIV8VV4As8O40]4− polyoxoanion with the shortest interatomic V⋯V distance of 3.02 Å and a diameter of ca. 11 Å. In the crystal of (NH4)4[V12As8O40(H2O)]·4H2O, the AsPOVs are joined by weak intercluster As⋯O interactions with the shortest As⋯O distance of 3.05 Å. The H2O molecules and NH4+ countercations reside in the voids between the polyoxoanions. The strong hydrogen bonds formed between the NH4+ ions and the terminal O atoms of the [H2O@VIV8VV4As8O40]4− polyoxoanion result in the formation of a 3D network. (NH4)4[V12As8O40(H2O)]·4H2O exhibits a distinct solubility in H2O, MeOH and DMF and shows an optical energy gap of 2.80 eV.
Zinc–AsPOV hybrids. A number of hydrothermally synthesised compounds based on the bis-transition metal-substituted AsPOVs of the type {M2V12As8O40}, which exhibit antiferromagnetic exchange interactions, were described. These hybrid polyoxoanions are formally derived from the α-{V14As8O42} polyoxoanion (discussed in Section 3.2.4) in which two {VO}2+ caps situated between the mutually opposite {As2O5} groups are replaced by two M2+ ions. This kind of incorporation of transition metal cations into the backbones of dilacunary α-heteroPOV shells was also observed in the case of the aforementioned “fully-reduced” [Cd2(en)2VIV12O40(GeOH)8]4− polyoxoanion110 (Fig. 10). One of these M2-decorated AsPOVs was found in [{Zn(enMe)2}2(enMe)2{Zn2V12As8O40(H2O)}]·4H2O123 (Table 3). The main structural motif of this hybrid compound is a dilacunary {V12As8O40} shell functionalised with two [Zn2(enMe)3]2+ complexes via Zn–O bonds (Fig. 13a). Each of two [Zn(enMe)2]2+ moieties is linked to the [H2O@Zn2VIV12As8O40]4− polyoxoanion by the enMe residuals through Zn–N bonds. Strong hydrogen bonds contribute to the formation of a 3D supramolecular array. The crystal structure of another similar compound {[Zn(dien)]2(dien)2[Zn2V12As8O40(0.5H2O)]}2·6H2O124 (Table 3) displays two crystallographically independent [0.5H2O@Zn2VIV12As8O40]4− polyoxoanions each of which is decorated with two [Zn(dien)]2+ complexes through dien connecting groups. The two resulting [Zn(dien)]2(dien)2[Zn2V12As8O40(0.5H2O)] constituents are linked to form a dimer by a weak Zn–O bond.
The compound [Zn(en)2]2[Zn2(bpe)2V12As8O40(H2O)] (Table 3) is composed of the [(bpe)2Zn2VIV12As8O40(H2O)]4− polyoxoanion [bpe = 1,2-bis(4-pyridyl)ethylene] and two [Zn(en)2]2+ countercations.125 This hybrid polyoxoanion based on the α-AsPOV is constructed from twelve {VO5} square pyramids, two square-pyramidal {ZnNO4} units and four handle-like {As2O5} groups. The Zn2+ ions introduced into the backbones of the AsPOV building block are coordinated by the bpe ligands through Zn–N bonds. The two terminal O atoms on the opposite sides of the eight-membered, {VO5}-composed ring of the [(bpe)2Zn2VIV12As8O40(H2O)]4− polyoxoanion are covalently connected to the [Zn(en)2]2+ complexes through Zn–O bonds. This compound with two structurally exposed pendant pyridyl rings of the bpe ligand could probably be of relevance for surface deposition studies.
The compounds [Zn(enMe)2]2[(4,4′-bipy)Zn2V12As8O40(H2O)], [Zn(en)2(H2O)][Zn(en)2(4,4′-bipy)Zn2V12As8O40(H2O)]·3H2O, [{Zn(en)3}2{Zn2V12As8O40(H2O)}]·4H2O·0.25bipy and [Zn2(en)5][{Zn(en)2}{(bpe)HZn2V12As8O40(H2O)}2]·7H2O composed of zinc(II) amine complexes and the “fully-reduced”, bis-zinc-substituted α-isomeric AsPOVs with compositions [H2O@Zn2VIV12As8O40]4− and its protonated [H2O@HZn2VIV12As8O40]3− form were reported.126 Crystalline samples of these inorganic–organic hybrid materials could only be obtained in alkaline solutions when the pH value was adjusted between 8 and 10, which allowed deprotonation of N-donor ligands and their easier coordination to the Zn2+ ions (Table 3). The different structural features of the organic ligands captured by the secondary Zn2+ ions were shown to have an effect on the formation and construction of the above compounds, thus resulting in the polyoxoanions that are packed in linear or staggered arrangements in the solid state. The crystal structure of [Zn(enMe)2]2[(4,4′-bipy)Zn2V12As8O40(H2O)] exhibits linear chains of the [(4,4′-bipy)Zn2V12As8O40(H2O)]4− polyoxoanions charge-balanced by discrete [Zn(enMe)2]2+ complexes occupying the interchain regions. The “fully-reduced” [H2O@Zn2VIV12As8O40]4− polyoxoanions are linked by bridging 4,4′-bipy ligands through Zn–N bonds. It was also found that [Zn(enMe)2]2[(4,4′-bipy)Zn2V12As8O40(H2O)] is electrocatalytically active in the reduction and oxidation of H2O2 and NO2− using bulk-modified carbon paste electrodes. The crystal structure of [Zn(en)2(H2O)][Zn(en)2(4,4′-bipy)Zn2V12As8O40(H2O)]·3H2O displays 1D winding chains of the [(4,4′-bipy)Zn2V12As8O40(H2O)]4− hybrid clusters functionalised with the [Zn(en)2]2+ complexes through Zn–O bonds; [Zn(en)2(H2O)]2+ complexes act as countercations. Similarly to the previous compound, the 4,4′-bipy ligands bridge the {Zn2V12As8O40} building blocks through Zn–N bonds. The Zn2+ cations are incorporated into the dilacunary-type AsPOV shell through Zn–O bonds. The extensive hydrogen bonding in the crystal lattice results in the formation of a 3D supramolecular architecture. The crystal structure of [{Zn(en)3}2{Zn2V12As8O40(H2O)}]·4H2O·0.25bipy is described as an eight-shaped chiral helical chain, where the “fully-reduced” [H2O@Zn2VIV12As8O40]4− hybrid polyoxoanions are covalently bridged by the [Zn(en)3]2+ complexes through Zn–N and Zn–O bonds, as shown in Fig. 13b. In contrast to the above-mentioned compounds, [Zn2(en)5][{Zn(en)2}{(bpe)HZn2V12As8O40(H2O)}2]·7H2O has a 2D layer structure with nanosized inner 1D rectangular cavities of 33.7 × 14.7 Å. These cavities are occupied by the lattice H2O molecules and [Zn2(en)5]4+ cations. The connectivity of the “fully-reduced” [H2O@HZn2VIV12As8O40]3− building blocks through the [Zn(en)2]2+ complexes and the bidentate bpe ligands (Fig. 13c), yielding a single-stranded (right- and left-handed) helical chain, resembles that found in the crystal structure of [Zn(en)2]2[Zn2(bpe)2V12As8O40(H2O)].125
Cadmium–AsPOV hybrids. Cd2+–en complexes were incorporated into the dilacunary β-{V12As8O40}-type structure through Cd–O bonds to form the “fully-reduced” [Cd2(en)2VIV12As8O40]4− polyoxoanion, which is charge-balanced by two [Cd(en)2]2+ complexes.127 This compound with composition [Cd(en)2]2[Cd2(en)2V12As8O40] was prepared under hydrothermal conditions (Table 3) and displays a 1D chain structure where neighbouring [Cd2(en)2VIV12As8O40]4− polyoxoanions are doubly bridged via [Cd(en)2]2+ groups. The interface between these hybrid building blocks is characterised by eight-membered rings involving Cd2+ ions from the TMC linkers and terminal and bridging O atoms from the connected polyoxoanions. Hydrogen bonds extend the 1D chains into a 3D supramolecular network.
Two other compounds, [Cd(enMe)3]2[(enMe)2Cd2V12As8O40(0.5H2O)]·5.5H2O and [Cd(enMe)2]2[Cd2(enMe)2V12As8O40(0.5H2O)], were found to contain α- and β-isomeric [Cd2(enMe)2VIV12As8O40(0.5H2O)]4− constituents, respectively (Table 3).128 Whereas the former compound can be viewed as an isolated (0D) inorganic–organic hybrid, the latter compound shows an infinite, 1D linear chain structure due to the [Cd(enMe)2]2+ linkages and is furthermore isomorphic with the above-mentioned example [Cd(en)2]2[Cd2(en)2V12As8O40],127 with the exception of organic ligands coordinated to the Cd(II) centres and a half H2O molecule encapsulated by the polyoxoanion shell. In both compounds, the [Cd(enMe)]2+ complexes are attached to the AsPOV building blocks through Cd–O bonds. According to the diffuse reflectance UV-vis spectra, these compounds are characterized by optical energy gaps of ca. 2 eV.
Zinc–AsPOV hybrid with a mixed V13/V14-structure. The compound [Zn(2,2′-bipy)3]4[(ppz){{Zn(tepa)}2ZnV13As8O41(H2O)}2][V14As8O42(0.5H2O)]2·4H2O (tepa = tetraethylenepentamine)124 displays two different types of AsPOV constituents, namely the Zn-monosubstituted [H2O@ZnVIV13As8O41]4− hybrid and the [0.5H2O@VIV14As8O42]4− polyoxoanion. The latter belongs to the class of {V14As8}-based compounds which are discussed in the next section. The ppz and tepa organic groups in this compound were in situ-formed from dien molecules used in the hydrothermal reaction (Table 3) and coordinate to the Zn2+ cations to result in the complexes [Zn2(ppz)]4+ and [Zn(tepa)]2+. The [Zn2(ppz)]4+ unit bridges the two neighbouring α-{V13As8}-type AsPOVs, each of which is decorated with two [Zn(tepa)]2+ moieties through Zn–O bonds (Fig. 14a). The Zn2+ ions of the [Zn2(ppz)]4+ complex are integrated into the backbones of the [{Zn(tepa)}2VIV13As8O41(H2O)]2− polyoxoanions to form [{Zn(tepa)}2ZnVIV13As8O41(H2O)], which are thus held together by ppz linkers via Zn–N bonds.
Cadmium–AsPOV hybrid. The “fully-reduced” [Cd(dien)VIV13As8O41(H2O)]4− polyoxoanion (Fig. 14b) with the seven-coordinate Cd2+ ion was hydrothermally isolated as the compound [Cd(dien)2]2[Cd(dien)V13As8O41(H2O)]·4H2O (Table 3) showing strong antiferromagnetic interactions between the spin-1/2 vanadyl {VO}2+ moieties.127 The other two hydrothermally prepared compounds [Cd(en)3][Cd(phen)(en)(H2O)2][Cd(en)V13As8O41(H2O)]·1.5H2O and [Cd(phen)2(en)]2[Cd(phen)V13As8O41(H2O)]·21H2O·phen (phen = 1,10-phenanthroline) feature the [Cd(en)VIV13As8O41(H2O)]4− and [Cd(phen)VIV13As8O41(H2O)]4− structures, where the Cd2+ ions coordinated by en and phen ligands are incorporated into the monolacunary α-type heteroPOV shells (Table 3).125 In contrast to [Cd(dien)V13As8O41(H2O)]4−, the antiferromagnetic [Cd(en)VIV13As8O41(H2O)]4− and [Cd(phen)VIV13As8O41(H2O)]4− hybrid polyoxoanions comprise six-coordinate Cd2+ ions. In all these Cd-containing compounds, the [Cd(dien)2]2+, [Cd(en)3]2+, [Cd(phen)(en)(H2O)2]2+, and [Cd(phen)2(en)]2+ complexes act as countercations.
Nickel–AsPOV hybrid. The “fully-reduced” [NiVIV13As8O41]4− polyoxoanion with the NiII-filled monolacunary POV structure was isolated as the compound {[V13As8NiClO41][Ni(en)2(H2O)][Ni(en)2]}{[Ni(en)2(H2O)2]0.5}·4H2O under hydrothermal reaction conditions (Table 3).129 Its crystal structure displays the host–guest [Cl@NiVIV13As8O41]5− building blocks linked to each other through Ni–O–As bonds to form a dimeric assembly (Fig. 14c). The latter is further connected to the neighbouring dimer via bridging [Ni(en)2]2+ complexes (corner-sharing Ni–Oterm–V interactions) to result in an infinite 1D chain. The [Cl@NiVIV13As8O41]5− structure can be described as being constituted of thirteen square-pyramidal {VO5} moieties, four handle-like {As2O5} groups, and one square-pyramidal {ClNiO4} entity with the strong Ni–Cl bonding interaction. The [Ni(en)2(H2O)]2+ and [Ni(en)2]2+ complexes and a half [Ni(en)2(H2O)2]2+ complex compensate the negative charge of this hybrid polyoxoanion. The compound exhibits antiferromagnetic properties.
Discrete AsPOVs. This class of AsPOVs offers a large number of discrete polyoxoanions. In 1991, Müller and Döring reported a series of the “fully-reduced” α-AsPOVs with the general formula [Y@V18–zAs2zO42]m− where Y = SO32−, SO42− or H2O and z = 4. These host–guest polyoxoanions were isolated as crystal solvent-free ammonium compounds (NH4)6[V14As8O42(SO3)], (NH4)6[V14As8O42(SO4)] and (NMe4)4[V14As8O42(H2O)].130 The authors highlighted that the V/As ratio, pH, and concentration of the reducing agents such as hydrazine sulfate (N2H6SO4), hydrazine chloride (N2H5Cl) und sodium dithionite (Na2S2O4) play an important role in the formation of these {Y@V14As8O42}-based compounds (Table 3) where the inner voids of the POV shells are occupied by statistically disordered small anionic or neutral guest (Y) species. Here, the D2d-symmetrical AsPOVs are formally derived from the corresponding [Y@V18O42]m− structures by replacing four {VIVO}2+ vanadyl groups in the latter with four {AsIII2O}4+ moieties.
Also in 1991, Huan et al. presented a spherical [(H2O)0.5@VIV14As8O42]4− polyoxoanion that formed as the compound (NMe4)4[V14As8O42(H2O)0.5] under hydrothermal conditions (Table 3).131 This “fully-reduced” AsPOV consists of fourteen condensed {VO5} square pyramids and four handle-like {As2O5} groups and is characterised by a α-{V14As8O42} shell of rhombicuboctahedral topology (Fig. 15a). This cluster shell encapsulates a disordered H2O guest molecule. The β-isomer (Fig. 15b) encapsulating a statistically disordered SO42− ion was identified in the hydrothermally prepared compound [NH2(CH2)4NH2]4[V14As8O42(SO4)]·(HSO4)2 (Table 3).132 In contrast to the α-isomer with D2d symmetry, the “fully-reduced” β-[SO4@VIV14As8O42]6− polyoxoanion exhibits D4h symmetry which results from the rotation of the three-membered vanadium oxide arc and two {As2O5} groups over an eight-membered ring in α-[VIV14As8O42]4− by 90° around the S4 axes (Fig. 15a and b). In the structure crystal the (C4N2H12)2+ piperazine cations, two HSO4− anions and terminal O atoms of the β-AsPOV building blocks are involved in strong hydrogen bonding interactions, thus generating a 3D network structure.
 | ||
| Fig. 15 Polyhedral representation of the discrete “fully-reduced” α-[VIV14As8O42]4− (a) and β-[VIV14As8O42]4− (b) polyoxoanions. Colour code: As, rose; O, red; VIVOx, sky-blue polyhedra. | ||
Other polyoxoanions with the same or a similar elemental composition were also reported. The antiferromagnetic compound [NH2(CH2CH2)2NH2]3[V14As8O42(SO4)]·6.5H2O was obtained by the hydrothermal reaction in acidic solution at pH = 3 (Table 3).133 Yang and colleagues showed that protonated [NH2(CH2CH2)2NH2]2+ amine molecules not only compensate the negative charge of the “fully-reduced” α-[SOVIV14As8O42]6− polyoxoanion but also assume space-filling and structure-directing roles. The strong hydrogen bonds between the AsPOV building blocks, organic amine molecules, and crystal water molecules result in the formation of a 3D supramolecular array. The “fully-reduced” β-[SO4@VIV14As8O42]6− isomer was synthesised as the compound (NH4)2(NMe4)4[V14As8O42(SO4)] under hydrothermal conditions.134 The hydrothermal synthesis and characterisation of two other compounds (H2en)3.5[V14As8O42(PO4)]·2H2O135 and (H2enMe)2[V14As8O42(H2O)]·3H2O136 based on the “fully-reduced” α-polyoxoanions with encapsulated PO43− and H2O guests, respectively, were described as well (Table 3).
Discrete AsPOV with rubidium countercations. The above series of discrete, host–guest AsPOVs also includes the “fully-reduced” [Cl@VIV14As8O42]5− polyoxoanion, which was shown to exhibit elongated square gyrobicupola topology that is also observed for [VIV16Ge4O42(OH)4]8− (Fig. 8a). This AsPOV pentaanion is the central component of the hydrothermally prepared compound Rb5[V14As8O42(Cl)]·2H2O with a chain-like structure.116 Interestingly, the [Cl@VIV14As8O42]5− polyoxoanion could only be isolated in the presence of Rb+ cations (Table 3) and not with Na+, K+ or Cs+.
Transformation of {V14As8} into {V6As8}-type AsPOV. It is worth mentioning that, in the general case, the [VIV14As8O42]4− polyoxoanion can be formally converted into the lower-nuclearity [VIV6As8O26]4− polyoxoanion by removal of the central eight-membered ring composed of edge-sharing {VO5} square pyramids.137 The “fully-reduced” [VIV6As8O26]4− structure thus consists of four handle-like {As2O5} groups and six distorted {VO5} square pyramidal units with the shortest VIV⋯VIV distance of ca. 4.5 Å. Furthermore, this AsPOV contains more As than V atoms, thus giving a high As/V ratio of 4/3, a very unusual situation in the structural chemistry of heteroPOVs discussed herein. The [VIV6As8O26]4− polyoxoanion crystallised under reducing conditions at room temperature as the compound (NnBu4)4[V6As8O26] (Table 3) and shows antiferromagnetic behaviour.
TMC–AsPOV hybrids without specific cation–anion interactions. The two antiferromagnetic compounds [Zn(2,2′-bipy)3]2[V14As8O42(H2O)]·4H2O and [Zn(2,2′-bipy)(dien)]2[V14As8O42(H2O)]·2H2O were prepared hydrothermally at pH = 7 using organic amines as reducing agents for the starting V2O5 material (Table 3).138 These compounds are composed of the “fully-reduced” α-[H2O@VIV14As8O42]4− polyoxoanions with approximate D2d symmetries and [Zn(2,2′-bipy)3]2+ and [Zn(2,2′-bipy)(dien)]2+ countercations, respectively. In contrast to [Zn(2,2′-bipy)3]2+, the [Zn(2,2′-bipy)(dien)]2+ complexes are involved in extensive hydrogen bonding interactions with the oxygen atoms of the AsPOVs, thus yielding a densely packed 3D network in the crystal lattice of the [Zn(2,2′-bipy)(dien)]2[V14As8O42(H2O)]·2H2O compound.
The nickel(II) and cobalt(II) analogues (2,2′-bipy)[Ni(2,2′-bipy)3]2[α-V14As8O42(H2O)]·3H2O and [Co(2,2′-bipy)3]2[α-V14As8O42(H2O)]·3H2O of the [Zn(2,2′-bipy)3]2[α-V14As8O42(H2O)]·4H2O compound were also hydrothermally synthesised (Table 3).139 Similarly, the inorganic–organic hybrid compounds [Cu(en)2]2[V14As8O42(H2O)]·2.5H2O, [M(1,10-phen)3]2[V14As8O42(H2O)0.5]·0.5H2O (M = Mn, Cd) and [Co(dien)2]2[V14As8O42(H2O)]·3.5H2O were obtained by pH-controlled hydrothermal syntheses.140 Notably, the α-[H2O@VIV14As8O42]4− polyoxoanions in the crystal structure of [Co(dien)2]2[V14As8O42(H2O)]·3.5H2O are linked by van der Waals forces into channels where the [Co(dien)2]2+ complexes and H2O molecules act as space-fillers.
The excitation and emission spectra of other hydrothermally prepared compounds [Zn(phen)3]2[V14As8O42(H2O)]·4H2O and [Cd(phen)3]2[V14As8O42(H2O)]·2H2O (Table 3) were reported that indicate fluorescence in the UV region with an emission peak at ca. 300 nm.141 Although the authors claimed that these compounds feature a new type of the “fully-reduced” [VIV14As8O42]4− polyoxoanion, namely the γ isomer with dV⋯V = 3.01–3.04 Å, their structures are in fact characterised by co-crystallised polyoxoanions of α and β types with occupation factors of 0.5. For the rotational isomerism, electronic structures and acidity/basicity properties of “fully-reduced” [VIV14E8O50]12− heteroPOVs and their chalcogenide-substituted [VIV14E8O42X8]12− derivatives (E = Si, Ge, Sn; X = S, Se, Te), see the theoretical work of Kondinski et al.142 For comparison, Fig. 16 illustrates the α-, γ-, and β-isomeric vanadium oxide skeletons of the {V14As8}-nuclearity AsPOVs.
 | ||
| Fig. 16 The “fully-reduced” vanadium oxide skeletons of α-/γ-/β-[VIV14As8O42]4− polyoxoanions. The {As2O} moieties are not shown. Colour code: O, red; VIVOx, sky-blue polyhedra. | ||
TMC–AsPOV hybrids with specific cation–anion interactions. A large series of {V14As8O42}-type polyoxoanions charge-balanced and covalently coordinated with various TMC complexes were presented by Yang, Xu, Wang, Peng, Das and coworkers. The inorganic–organic hybrid compounds with the α-[VIV14As8O42]4− building blocks pillared by Zn(II) complexes [Zn(en)2][(H2O)Zn(en)2V14As8O42(H2O)]·H2O, [{(H2O)Zn(1,10-phen)2}2{V14As8O42(H2O)}]·4H2O, [{(H2O)Zn(2,2′-bipy)2}{Zn(2,2′-bipy)2}V14As8O42(H2O)0.5]2[{(H2O)Zn(2,2′-bipy)2V14As8O42(H2O)0.5}2{Zn(2,2′-bipy)2}2]·3H2O, and [{(H2O)Zn(4,4′-bipy)}2{V14As8O42(H2O)}]·2H2O were all synthesised under hydrothermal conditions.143 These compounds display differing extended structures as well as different coordination modes of the organodiamine ligands (en, 1,10-phen, 2,2′-bipy, and 4,4′-bipy) used in the synthesis reactions (Table 3). Notably, the formation of the crystalline products was shown to be strongly dependent on the acidic pH range (3–6), which is quite different from the pH (alkaline media) for reactions resulting in the TMC-supported GePOVs, and was seemingly not influenced by the reaction temperatures in the range 130–170 °C. The layered crystal structure of [Zn(en)2][(H2O)Zn(en)2V14As8O42(H2O)]·H2O features the [(H2O)Zn(en)2V14As8O42(H2O)]2− anion, in which a single [Zn(en)2(H2O)]2+ moiety is attached to a terminal O atom from the eight-membered ring of the AsPOV, and the [Zn(en)2]2+ complex is the counteraction. The [{(H2O)Zn(1,10-phen)2}2{V14As8O42(H2O)}]·4H2O compound shows the “fully-reduced” [VIV14As8O42]4− polyoxoanion whose central eight-membered ring is doubly decorated with the [(H2O)Zn(1,10-phen)2]2+ complexes through covalent Zn–O bonds. The crystal structure is, furthermore, characterised by a 2D supramolecular array formed due to an extended hydrogen bond network. The crystal structure of [{(H2O)Zn(2,2′-bipy)2}{Zn(2,2′-bipy)2}V14As8O42(H2O)0.5]2[{(H2O)Zn(2,2′-bipy)2V14As8O42(H2O)0.5}2{Zn(2,2′-bipy)2}2]·3H2O displays two crystallographically independent motifs, namely [{(H2O)Zn(2,2′-bipy)2V14As8O42(H2O)0.5}2{Zn(2,2′-bipy)2}2] and [{(H2O)Zn(2,2′-bipy)2}{Zn(2,2′-bipy)2}V14As8O42(H2O)0.5]. The structure of the former, neutral dimer consists of two [(H2O)Zn(2,2′-bipy)2V14As8O42(H2O)0.5]2− hybrids, in which each AsPOV building block is coordinated with one [(H2O)Zn(2,2′-bipy)2]2+ complex, and two [Zn(2,2′-bipy)2]2+ complexes function as bridging groups between these two dianions. The structure of another neutral hybrid compound [{(H2O)Zn(2,2′-bipy)2}{Zn(2,2′-bipy)2}V14As8O42(H2O)0.5] consists of the [(H2O)Zn(2,2′-bipy)2]2+ and [Zn(2,2′-bipy)2]2+ moieties covalently attached to the AsPOV building block via Zn–O bonds. In the structure, the [(H2O)Zn(2,2′-bipy)2]2+ complex is involved in bonding to a terminal O atom from the eight-membered ring of [VIV14As8O42]4− and the [Zn(2,2′-bipy)2]2+ complex to that of a {VO5}-composed trimer capping the AsPOV ring. The crystal structure of [{(H2O)Zn(4,4′-bipy)}2{V14As8O42(H2O)}]·2H2O displays a 2D network where the adjacent AsPOVs are linked by two [(H2O)Zn(4,4′-bipy)]2+ fragments through covalent Zn–O bonds. Thus, each polyoxoanion is surrounded by four bridging Zn(II) complexes coordinated to the terminal O atoms of the eight-membered vanadium oxide ring. The 2D network of this compound is further expanded into a 3D supramolecular structure via hydrogen bonds between water molecules and terminal oxygen positions of [VIV14As8O42]4−. According to cyclic voltammograms, the above-described compounds exhibit quasi-reversible redox behaviour.
The extended solid-state structures of the hydrothermally prepared compounds [Zn(2,2′-bipy)2]2[V14As8O42(H2O)]·H2O (Fig. 17a), [Cd(2,2′-bipy)3][Cd(dien)V14As8O42(H2O)], and [Ni(en)2]3[V14As8O42(HPO3)]·4H2O (Table 3) display the “fully-reduced” α-[VIV14As8O42]4− polyoxoanions interconnected through the corresponding TMC complexes [Zn(2,2′-bipy)2]2+, [Cd(dien)]2+, and [Ni(en)2]2+, respectively.144 The Zn(II)- and Cd(II)-containing compounds organise into 1D chain structures and are characterised by overall antiferromagnetic coupling. In contrast, the Ni(II)-containing compound exhibits a 2D structure and produces no signal for VIV ions in the EPR spectrum at room temperature.
The antiferromagnetic compound [Ni(en)2]3[V14As8O42(SO4)]·4.5H2O with a 2D layer structure was prepared under hydrothermal conditions (Table 3) and reveals strong covalent attachment between the adjacent α-[SO4@VIV14As8O42]6− polyoxoanions through V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–Ni–O
O–Ni–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) V connectivities.145 A 2D network is formed as POV is bound to four neighbouring polyoxoanions via an octahedrally coordinated Ni(II) centre.
V connectivities.145 A 2D network is formed as POV is bound to four neighbouring polyoxoanions via an octahedrally coordinated Ni(II) centre.
The compounds [{Ni(en)2}4(4,4′-bipy)4{Ni(H2O)2}]2[V14As8O42(NO3)]4·16H2O and [Ni(en)2(H2O)2]2[{Ni(en)2(H2O)}2V14As8O42(NO3)][{Ni(en)2}V14As8O42(NO3)]·6H2O (Table 3) were hydrothermally prepared and showed high-dimensional organic–inorganic hybrid nanostructures.146 The compound bearing 4,4′-bipy ligands consists of two bridging [{Ni(en)2}4(4,4′-bipy)4{Ni(H2O)2}]10+ fragments and four α-[NO3@VIV14As8O42]5− polyoxoanions which are joined through Ni–O bonds to form a pillared box-like structure with a cavity of ca. 600 Å3 (Fig. 18). In contrast to the previous compound, the compound [Ni(en)2(H2O)2]2[{Ni(en)2(H2O)}2V14As8O42(NO3)][{Ni(en)2}V14As8O42(NO3)]·6H2O is based on the “fully-reduced” β-[NO3@VIV14As8O42]5− polyoxoanions. Its crystal structure shows three crystallographically independent motifs: (i) bis-{Ni(en)2(H2O)}-decorated AsPOV anion [{Ni(en)2(H2O)}2V14As8O42(NO3)]−; (ii) 1D chain polymer consisting of [{Ni(en)2}2V14As8O42(NO3)]− monoanions; (iii) [Ni(en)2(H2O)2]2+ complexes.
The hydrothermal synthesis of ‘metal-controlled’ inorganic–organic self-assemblies with compositions [Ni(enMe)3]4[Ni(enMe)2][V14As8O42(NO3)]2·8H2O and [Co(en)3][Co(en)2V14As8O42(H2O)]·16H2O (Table 3) was reported. These antiferromagnetic compounds are based on the “fully-reduced” α-[VIV14As8O42]4− polyoxoanions accommodating NO3− and H2O as guests.125 The crystal structure of the former AsPOV consists of [Ni(enMe)2{V14As8O42(NO3)}2]8− dimers, four [Ni(enMe)3]2+ countercations and lattice H2O molecules. The [Ni(enMe)2{V14As8O42(NO3)}2]8− dimer is constructed from two “fully-reduced” [NO3@VIV14As8O42]5− moieties linked through the bridging [Ni(enMe)2]2+ complex. The crystal structure of the latter compound is characterised by 1D chains consisting of the [Co(en)2V14As8O42(H2O)]2− hybrids, [Co(en)3]2+ countercations and lattice H2O molecules. The [Co(en)2]2+ complexes act here as bridging ligands for the neighbouring AsPOVs.
Another Co(II)-containing compound with the formula [Co(2,2′-bipy)2]2[V14As8O42(H2O)]·H2O (Fig. 17b) and a tubular structure was prepared hydrothermally in acidified solution (Table 3).147 The crystal structure of this solid being stable up to ca. 370 °C shows closed helical chains of {[Co(2,2′-bipy)2][VIV14As8O42(H2O)]}∞ hybrid building blocks where the [Co(2,2′-bipy)2]2+ complexes covalently link the α-isomeric AsPOVs through Co–O bonds.
A number of high-dimensional hybrid materials based on the α-[VIV14As8O42]4− building block, namely [M(bbi)2]2[V14As8O42(H2O)] (M = Co, Ni, Zn) and [Cu(bbi)]4[V14As8O42(H2O)] were synthesised under hydrothermal conditions (pH of ca. 5.0) where oxalic acid can act as a reducing agent for the VV source (Table 3).148 These compounds containing the soft N-donor 1,1′-(1,4-butanediyl)bis(imidazole) (= bbi, C10H14N4) extend a series of POV-templated structures including, e.g., that of the [H4V18O46(SiO)8(dab)4(H2O)]·4H2O compound bearing the bidentate dab ligand.97 The isostructural [M(bbi)2]2[V14As8O42(H2O)] (M = Co, Ni, Zn) compounds with antiferromagnetic properties are characterised by a binodal (4,6)-connected 2D network structure, Schläfli symbol (34·42)(34·44·54·63)2, where each of the neighbouring AsPOVs is covalently coordinated to four bridging [M(bbi)2]2+ complexes through M–O bonds. The coordination modes of the bbi ligands allow them to connect these four M2+ ions in such a manner that each polyoxoanion is located within a closed {M(bbi)}4 ring. In the structure of the compound [Cu(bbi)]4[V14As8O42(H2O)] a 3D network is observed made up of the AsPOVs interacting covalently with the bbi-bridged Cu+ cations from the adjacent wave-like chains of the [Cu(bbi)]n moieties. The latter surrounding each polyoxoanion up and down results in the formation of 1D ladder-like [Cu(bbi)]+ double chains.
The compound [Cu(en)2]3[V14As8O42(CO3)]·10H2O with a 2D network structure was prepared hydrothermally (Table 3) and contains α-[CO3@VIV14As8O42]6− with a carbonate ion in the inner void of the polyoxoanion.149 In the solid state, each AsPOV is surrounded by six [Cu(en)2]2+ bridging groups with Cu–Oterm–V linkages (Fig. 17c).
Lanthanoid–AsPOV hybrids. The first representatives of AsPOVs with covalently attached aqua-lanthanoid(III) complexes were reported in 2009.150 Arumuganathan and Das isolated three isostructural [{Ln(H2O)6}2V14As8O42(SO3)]·8H2O compounds (Ln = LaIII, SmIII, and CeIII) from aqueous reaction solutions at room temperature using (NH4)6[V14As8O42(SO3)] and Ln(NO3)3·6H2O as starting materials (Table 3). The main structural motif of the all-inorganic {V14As8}-type compounds represents the “fully-reduced” β-[VIV14As8O42]4− polyoxoanion with approximate D2d symmetry, which encapsulate a sulfite anion. As was demonstrated by thermal analysis and mass spectrometry experiments, the aqua-Ln(III)-capped AsPOVs release gaseous SO2 at temperatures 520–580 °C, whereas the (NH4)6[V14As8O42(SO3)] precursor releases SO2 already at 480–520 °C. The crystal structures of these compounds display 2D layered coordination polymers where each of six [Ln(H2O)6]3+ complexes surrounding the [SO3@VIV14As8O42]6− polyoxoanion through one covalent Ln–Oterm–V bond coordinates to two other adjacent AsPOVs. Thus, the aqua-Ln(III) complexes can indeed link to AsPOVs, which resulted in the formation of extended structures where LaIII ions are nine-fold coordinated and reside in a monocapped square-antiprismatic coordination environment. Magnetic susceptibility studies performed for the samples with the 4f0-LaIII, 4f5-SmIII and 4f1-CeIII ions indicated that [{Ln(H2O)6}2V14As8O42(SO3)]·8H2O are antiferromagnetically coupled materials, with no significant coupling between the lanthanide(III) ions and the POV.
Discrete AsPOV with potassium countercations. The first synthesised AsPOV dates back to 1988, when Müller and Döring published the compound K6[V15As6O42(H2O)]·8H2O which was prepared by reduction of vanadate with hydrazinium sulfate in the presence of As2O3 in aqueous solution at 85 °C (Table 3).151 This compound is composed of the “fully-reduced” [H2O@VIV15As6O42]6− polyoxoanion with crystallographic D3 symmetry and six K+ countercations. The “hydrated” potassium ions provide the networking of the AsPOVs in the crystal structure (rhombohedral cell; for hexagonal cell, see K6[V15As6O42(H2O)]·6H2O).152 The [H2O@VIV15As6O42]6− polyoxoanion with an encapsulated H2O molecule (Fig. 19a) is the first member of the series of compounds with general formula [V18–zAs2zO42] (z = 3). The structural motif of [H2O@VIV15As6O42]6− is derived from the [V18O42]12− structure by replacing three {VO5} units by three handle-like {As2O5} groups (for comparison, see [VIV15Si6O42(OH)6]6− in Fig. 6c). This polyoxoanion thus consists of fifteen distorted {VO5} square pyramids that are interlinked through the basal O vertices and edges and of six {AsO3} trigonal pyramids. Interestingly, Müller and Döring noticed that this prominent AsPOV “may be regarded as a model for species that are formed during poisoning of the V2O5catalyst (which contains VIVcenters) by arsenic”.151
TMC–AsPOV hybrids without specific cation–anion interactions. The compound [Zn(H2O)4]2[H2V15As6O42(H2O)]·2H2O was obtained under hydrothermal conditions (Table 3) and is composed of the doubly protonated [H2O@H2VIV15As6O42]4− polyoxoanion and two [Zn(H2O)4]2+ countercations.153 In the structure, these two constituents are held together by hydrogen O–H⋯Oterm bonds, which results in the formation of a 3D network structure. In studies of third-order nonlinear optical (NLO) properties of [Zn(H2O)4]2[H2V15As6O42(H2O)]·2H2O the compound exhibited strong nonlinear absorption.
TMC–AsPOV hybrids with specific cation–anion interactions. The structures of two hydrothermally synthesised compounds [Zn(en)2][Zn(en)2(H2O)2][{Zn(en)(enMe)}V15As6O42(H2O)]·4H2O and [Zn2(enMe)2(en)3][{Zn(enMe)2}V15As6O42(H2O)]·4H2O (Table 3) consist of the [{Zn(en)(enMe)}VIV15As6O42(H2O)]4− (Fig. 19b) and [{Zn(enMe)2VIV15As6O42(H2O)}]4−hybrid polyoxoanions whose AsPOV shells are coordinatively functionalised with Zn2+ complexes through Zn–Oterm–V bonds.154 Charge neutrality of the compounds is achieved by cationic {[Zn(en)2][Zn(en)2(H2O)2]}4+ and [Zn2(enMe)2(en)3]4+ complexes. By contrast, a [{{Zn(en)2}2V15As6O42(H2O)}2{Zn(en)2}]2− dimer in another hydrothermally prepared compound (Hen)2[{{Zn(en)2}2V15As6O42(H2O)}2{Zn(en)2}]·3H2O (Table 3) is charge-balanced by two singly protonated Hen+ cations.155 The crystal structure of the latter compound shows two [H2O@VIV15As6O42{Zn(en)2}2]2− building blocks which are joined by a bridging [Zn(en)2]2+ unit through Zn–O bonds.
The “fully-reduced” [H2O@VIV15As6O42]6− polyoxoanions in the crystal structures of the antiferromagnetic compounds [Zn2(dien)3(H2O)2]0.5[{Zn2(dien)3}V15As6O42(H2O)]·2H2O (Fig. 19c, Table 3) and [Ni(2,2′-bipy)3]2[{Ni(en)2}V15As6O42(H2O)]·9.5H2O are interlinked by [Zn2(dien)3]2+ and [Ni(en)2]2+ moieties to form 1D helical chains and 1D infinite straight chains, respectively.156 The 1D helical chains are involved in extensive hydrogen bonding interactions with discrete [Zn2(dien)3(H2O)2]4+ complexes, thus generating a 2D network structure; a further expansion of the hydrogen bond pattern involving [Zn2(dien)3]4+ bridging units results in a 3D supramolecular array. The 1D chains based on the [H2O@VIV15As6O42{Ni(en)2}]4− polyoxoanions have been reported as possessing molecular recognition ability for the chiral [Ni(2,2′-bipy)3]2+ guests.
The hydrothermally prepared compound [Co(enMe)2]3[V15As6O42(H2O)]·2H2O (Fig. 19d and Table 3) is characterised by a 2D structure where each [H2O@VIV15As6O42]6− polyoxoanion is coordinated by six [Co(enMe)2]2+ moieties which in turn link the neighbouring AsPOVs to each other through Co–Oterm–V bonds.156 The crystal structure of the hydrothermally synthesised compound [Co(en)3][{Co(en)2}2V15As6O42]·4H2O (Table 3) shows an 1D infinite chain structure generated by Co–Oapical–V linkages.157 The neighbouring [VIV15As6O42]6− polyoxoanions are covalently linked through two six-coordinate cobalt(II) ions of μ2-{CoII(en)2}2+ moieties; isolated [CoII(en)3]2+ cations reside in interchain regions. This “fully-reduced” AsPOV exhibits weak antiferromagnetic exchange interactions between the vanadyl moieties.
A {V15As6}-type polyoxoanion with a higher degree of protonation was found in the hydrothermally synthesised compound [Cu(en)2]1.5[H3V15As6O42(H2O)]·3H2O (Table 3).158 In the crystal structure, the “fully-reduced” [H2O@H3VIV15As6O42]3− polyoxoanions are bridged by [Cu(en)2]2+ moieties leading to formation of a 1D sinusoidal chain (Fig. 19e). The series of protonated [H3VIV15As6O42]3− and [H2VIV15As6O42]4−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 polyoxoanions was further expanded by a [HVIV15As6O42]5− polyoxoanion as observed in the compound [Cu(enMe)2]2.5[HV15As6O42(H2O)]·2H2O exhibiting antiferromagnetic properties.136 This compound was prepared hydrothermally (Table 3) and shows a brick-wall-like 2D layer structure where the “fully-reduced” [H2O@HVIV15As6O42]5− polyoxoanions are doubly-bridged by the [Cu(enMe)2]2+ moieties.
153 polyoxoanions was further expanded by a [HVIV15As6O42]5− polyoxoanion as observed in the compound [Cu(enMe)2]2.5[HV15As6O42(H2O)]·2H2O exhibiting antiferromagnetic properties.136 This compound was prepared hydrothermally (Table 3) and shows a brick-wall-like 2D layer structure where the “fully-reduced” [H2O@HVIV15As6O42]5− polyoxoanions are doubly-bridged by the [Cu(enMe)2]2+ moieties.
{O3AsPh}/{O4AsPh}-functionalised AsPOVs. The vanadium oxide compounds containing phenylarsonate (O3AsPh)2− moieties were first described in the early 1990s. The antiferromagnetic compound [V2O4(HO3AsPh)]·H2O with a layered mixed-valent (VV/VIV) structure was hydrothermally synthesised and characterised by Huan et al.,160 while the compound displaying a phenylarsonate POV structure of higher nuclearity was described by Zubieta and colleagues161,162 The mixed-valent [H2{VIV2VV4O10(O3AsPh)6}]2− polyoxoanion was isolated as the compound (NnBu4)2[H2{V6O10(O3AsPh)6}]·2H2O (Table 3).161
Later on, the solvothermally prepared compounds (H7O3)2(NnBu4)2[(MeOH)2V12O14(OH)4(O3AsPh)10]·H2O and (NEt4)2[V12O12(OH)2(H2O)4(O3AsPh)10(HO3AsPh)4]·6H2O (Table 3) exhibiting, respectively, the nanoscopic organoarsonate POV cages with compositions [(MeOH)2@V12O14(OH)4(O3AsPh)10]4− (with approx. D2h symmetry) and [(H2O)2@V12O12(OH)2(H2O)2(O3AsPh)10(HO3AsPh)4]2− that cannot be derived from the {V18O42} archetype.162 The “fully-reduced” [(MeOH)2@VIV12O14(OH)4(O3AsPh)10]4− polyoxoanion is built up of edge- and corner-sharing {VO5} square pyramids, phenylarsonate tetrahedra and square pyramids and can be regarded as being composed of two {VIV4O5(O3AsPh)} fragments bridged by two {VIV2O2(OH)2(O3AsPh)4}6− moieties. The dianionic, “fully-reduced” AsPOV [(H2O)2@VIV12O12(OH)2(H2O)2(O3AsPh)10(HO3AsPh)4]2− consists of {VO5} square pyramids, {VO6} octahedra and phenylarsonate tetrahedra and square pyramids. The structure of this AsPOV is characterised by two {VIV5O6(O3AsPh)3}2+ fragments bridged by two {VIV(OH)(H2O)(O3AsPh)2(HO3AsPh)2}3− moieties. The geometric equivalence of the {AsPh}4+ groups to {VO}3+ by virtue of the nearly identical AsV–O and VV–O bond lengths was stressed by Khan and Zubieta.
In 2011, Zhang and Schmitt reported a unique series of symmetrical nanoscopic vanadium oxide supramolecular coordination cages functionalised with phenylarsonate ligands (Fig. 21) and exploited the topologies of their hollow structures together with the template effects of the octahedral {Xz(H2O)6−z} guest assemblies (X = Br, Cl and z = 2, 4, 6).163 Such “fully-reduced” VIV, mixed-valent VV/VIV and fully-oxidised VV AsPOVs characterised by the high-nuclearity {V16As8}, {V16As10}, {V20As8} and {V24As8} cages were found in the blue compounds (HNEt3)2[{Br2(H2O)4}(VIVO)16(OH)8(O4AsPh)2(O3AsPh)8]·6MeCN and (HNEt3)2[{Cl2(H2O)4}(VIVO)16(OH)8(O4AsPh)2(O3AsPh)8]·2H2O as well as the green compounds H5[{Cl4(H2O)2}(VVO)16O16(O3AsPh)8]Cl·4H2O·3MeCN, [{Cl4(H2O)2}(VVO)16(VIVO)4O16(OH)4(O3AsPh)8]·7H2O·3MeCN and H10[{Cl6}(VVO)16(VIVO)8O24(O3AsPh)8]Cl4·10H2O·2MeCN (Table 3). Their structures contain four- ({O3AsPh}) or five-fold ({O4AsPh}) coordinated AsV centres. The isomeric, “fully-reduced” {V16As10} cages in the blue compounds consist of sixteen {VO5} square-pyramids and ten fully deprotonated phenylarsonate ligands. Their AsPOV building block represents a modified [V18O42]12− structure in which two {VO5} caps on one diagonal are replaced with two {O4AsPh} moieties and two groups by four {O3AsPh} moieties slice the structure horizontally to isolate an eight-membered {VO5}-belt (Fig. 21, top). The key structural difference between the {V16As10} cages accommodating octahedral {Br2(H2O)4} and {Cl2(H2O)4} guest templates in their inner voids is that the two convex {VIV4O5(O3AsPh)} moieties in the Cl-containing AsPOV are rotated by 45° (isomer 2). This structural change is the result of the considerably different ionic radii of Br− and Cl− ions and the energies of V⋯Br and V⋯Cl interactions. The encapsulated symmetrical {Br2(H2O)4} and {Cl2(H2O)4} octahedra with the Br− and Cl− ions situated in the apical positions show Br⋯H2O and Cl⋯H2O distances ranging from 3.13 to 3.36 Å and from 3.03 to 3.18 Å, respectively. In addition, Br⋯Br distance of 5.28 Å is slightly shorter than the Cl⋯Cl distance of 5.31 Å. The intramolecular V⋯Br and V⋯Cl separations are between 3.47 and 3.51 Å. It was concluded that the nature and geometry of these octahedral guest assemblies determines the nuclearity, size, and topology of the produced organoarsonate POV cages.
The fully-oxidised {V16As8}- and mixed-valent {V20As8}- and {V24As8}-type building blocks (Fig. 21, bottom) of the above-mentioned green compounds were synthesised using Dy(NO3)3·nH2O (n ≈ 6), where presumably nitrate acts as an oxidant, different quantities of which was shown to influence the nuclearity of these AsPOV cages and the formal oxidation states of their V atoms. Thus, a gradual increase in the amount of the oxidant led ultimately to the fully-oxidised {V16As8}-nuclearity cage. The molecular structures of these three AsPOVs are constructed of sixteen, twenty and twenty-four edge- and corner-sharing {VO5} square-pyramids, respectively. Each POV shell is ligated by eight phenylarsonate groups. The {V16As8}-toroid cage encapsulates an octahedral {Cl4(H2O)2} guest template, in which the closest Cl⋯Cl/Cl⋯H2O distances amount to 3.97 Å/3.41 Å. The intramolecular V⋯Cl separations range from 2.82 Å to 3.02 Å. The {V20As8} cage can be seen as the toroidal structure of {V16As8} decorated with two {(VIVO)2(μ2-OH)2} units and is stabilised by the {Cl4(H2O)2} assembly as well. The Cl⋯Cl and Cl⋯H2O distances in this {Cl4(H2O)2} octahedron are 3.98 Å and 3.24–3.80 Å, respectively. The closest intramolecular V⋯Cl distances lie in the range 2.81–3.10 Å. The two encapsulated H2O molecules and the V ions of the {(VIVO)2(μ2-OH)2} entities are involved in V⋯Owater interactions at a distances of 2.36 Å. The {V24As8} cage encapsulates a {Cl6} aggregate with dCl⋯Cl = 3.91–4.01 Å the vertices of which are capped by six square {V4O8} fragments with electrophilic inner and nucleophilic outer environments. The closest V⋯Cl separations are in the range 2.94–3.033. The Archimedean body {V24} polyhedron (14 faces, 36 edges, 24 vertices) deduced from the mixed-valent {(VVO)16(VIVO)8O24(O3AsPh)8} fragment that can be regarded as a Keplerate displays a truncated octahedral topology. The {V24} polyhedron in the {V24As8} cage can thus be classified as one of the thirteen Archimedean solids.164 The vanadium skeletons of some of the discussed organoarsonate POV cages are illustrated in Fig. 22.
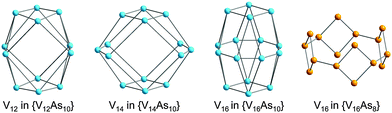 | ||
| Fig. 22 Topological representations of the vanadium skeletons deduced from some high-nuclearity organoarsonate POV cages represented in Fig. 21 and 23. Colour code: VIV, sky blue; VV, light orange. | ||
{O3AsC6H4-4-NH2}-functionalised AsPOVs. The group of Schmitt also reported two nanoscopic cages with compositions [V14O16(OH)8(O3AsC6H4-4-NH2)10]4− (Fig. 23), which shows a defect AsPOV structure from the {V16As10}-nuclearity cage (isomer 1, Fig. 21), and [V10O18(O3AsC6H4-4-NH2)7(DMF)2]5−.165 The compounds based on these AsPOVs were isolated from the condensation reactions under reducing conditions in aqueous solution (Table 3). The different nature of acids (HCl and HNO3) used in the synthesis of these compounds strongly influenced the nuclearity of the cages, resulting in the {V14As10}- and {V10As7}-type polyoxoanions, respectively. In contrast to the {O3AsPh}/{O4AsPh}-functionalised AsPOVs, the POV shells of these polyoxoanions are decorated with terminal (4-aminophenyl)arsonate ligands {O3AsC6H4-4-NH2}. Their skeleton structures are composed exclusively of {VO5} square-pyramids. Whereas the electrophilic void of the “fully-reduced” {V14As10} cage (dimensions: 5.8 × 5.9 × 5.9 Å) with an almost ideal cubic arrangement of the As atoms contains two Cl− ions and four H2O molecules forming a stabilising octahedral assembly, the mixed-valent asymmetric {V10As7} cage is characterised by a hexagonal packing due to the amine functionalities engaged in hydrogen bonds in the structure.
The [V12O14(OH)4(O3AsC6H4-4-NH2)10]4− polyoxoanion with the VIV/arsonate nuclearity [12(VO5):10(O3AsR)], lower than that [14(VO5): 10(O3AsR)] of the above-mentioned [V14O16(OH)8(O3AsC6H4-4-NH2)10]4− polyoxoanion, was isolated as the compound Na4(H2O)10[{V12O14(OH)4(H2O)3(O3AsC6H4-4-NH2)10}]·1.5DMF·1.25H2O (Table 3) from a condensation reaction involving p-arsanilic acid in a H2O/dimethylformamide (DMF) mixture.166 The main structural difference between these two AsPOVs (Fig. 22) is the number of the {VO5} square pyramids sandwiched between two tetranuclear {LVIV4O12} subunits where L is the (4-aminophenyl)arsonate ligand. The {V14As10} cage comprises two separated hydroxy-bridged {O4VIV(OH)2VIV(OH)2VIVO4} trimers, whilst the {V12As10} cage has only two separated, partially hydrated {O4VIV(OH)2VIVO4} dimers. The {VO5} square pyramids in the belt of each of these molecular cages are connected to two tetranuclear {LVIV4O12} subunits via additional eight (4-aminophenyl)arsonate ligands (Fig. 23). The synthesis and structural characterisation of the compound Na5[V5O9(O3AsC6H4-4-NH2)4]·20.5H2O·3DMF with the low-nuclearity inorganic–organic calix-type building block [V5O9(O3AsC6H4-4-NH2)4]5− comprising mixed-valent VV/VIV atoms were described as well (Table 3).166 The preliminary magnetic studies performed for these two compounds showed the presence of antiferromagnetic exchange interactions and S = 0 ground states.
An example of the {O3AsC6H4-4-NH2}-functionalised AsPOV with a quasi-planar, polycyclic-type structure was also reported. The fully-oxidised [VV10O24(O3AsC6H4-4-NH2)3]4− (Fig. 24a) polyoxoanion was isolated as the compound (NnBu4)2(NH4)2[V10O24(O3AsC6H4-4-NH2)3] (Table 3).161 The structure of this AsPOV can be regarded as a [V9O21(O3AsC6H4-4-NH2)3]3− toroid that encloses a {VO3}− unit in the centre of the wheel. The most unique feature of [VV10O24(O3AsC6H4-4-NH2)3]4− is that it contains a wheel-type [V7O24]13− substructure (Fig. 24b). Remarkably, the structure of this heptanuclear [V7O24]13− polyoxoanion displays striking similarities to the Anderson-type structures, e.g. of [TeM6O24]6−,167 [M7O24]6− (M = Mo, W),168 and [Bi7I24]3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 169 (Fig. 24c). The overall composition of [VV10O24(O3AsC6H4-4-NH2)3]4− formally consists of a double layer of polyhedra (bicapped Anderson type), with the one being composed of [V7O24]13− and the second, of a [V3(RAsO3)3]9+ ring. Another interesting feature is that it can be reversibly reduced by one electron to give the green-brown compound with composition [V10O24(O3AsC6H4-4-NH2)3]5−. According to electrochemical and magnetic measurements, this compound as well as the aforementioned (NnBu4)2[H2{V6O10(O3AsPh)6}]·2H2O161 are characterised by extensive electron storage and coupled electron–proton transfer processes.
169 (Fig. 24c). The overall composition of [VV10O24(O3AsC6H4-4-NH2)3]4− formally consists of a double layer of polyhedra (bicapped Anderson type), with the one being composed of [V7O24]13− and the second, of a [V3(RAsO3)3]9+ ring. Another interesting feature is that it can be reversibly reduced by one electron to give the green-brown compound with composition [V10O24(O3AsC6H4-4-NH2)3]5−. According to electrochemical and magnetic measurements, this compound as well as the aforementioned (NnBu4)2[H2{V6O10(O3AsPh)6}]·2H2O161 are characterised by extensive electron storage and coupled electron–proton transfer processes.
The discovery of the seminal [H2O@VIV15As6O42]6− polyoxoanion151 that was shown to act as a textbook example for quantum spin frustration and as a qubit with relatively long coherence lifetimes paved the way for studies focussed on the optical, electronic and magnetic properties of other iso- and heteroPOVs. The nanoscale magnetism of this {V15}-type AsPOV with a symmetrical layer structure of spin centres and spin frustration effects in the central VIV triangle is now well-documented. The [H2O@VIV15As6O42]6− polyoxoanion is a low-spin (spin-1/2) molecular nanomagnet and its characteristics were explored extensively: non-adiabatic Landau–Zener transitions,170 low-energy spin excitations by elastic neutron scattering study of K6[V15As6O42]·9D2O,171 low-energy excitations from proton NMR and μSR,172 adiabatic Landau–Zener–Stückelberg transitions with or without dissipation,173 mechanism of ground-state selection,174 local spin moment configuration determined by NMR,175 static magnetisation at ultra-low temperatures,176 quantum oscillations,177 and direct spin-phonon transitions.178 In general, such AsPOVs show extraordinary magnetic properties and can be used as models to study different fundamental phenomena as e.g. spin frustration179 and frustrated spin coupling, spin-phonon bottlenecks and butterfly hysteresis,180 Dzyaloshinsky–Moria interactions and other splitting effects,181 molecular-scale switching,182 quantum-spin tunnelling, spin coherence and low-temperature spin relaxation processes.183
3.3 Polyoxovanadatoantimonates (SbPOVs)
{V14Sb8}-type polyoxoanions. The “fully-reduced” α-[H2O@VIV14Sb8O42]4− polyoxoanion, with rhombicuboctahedral topology and isostructural to α-[VIV14As8O42]4− (Fig. 15a), was isolated as the compound [(H2en)2{V14Sb8O42(H2O)}]·(en)·4H2O under hydrothermal conditions184 (Table 4). Its crystal structure shows a 1D double-chain in which the sphere-like α-isomeric SbPOVs are interlinked through non-bonding Sb⋯O contacts of 2.74 and 2.79 Å. The intermolecular N–H⋯O hydrogen bonds between the terminal oxygen atoms of the SbPOVs and the hydrogen atoms of ethylenediamine molecules were observed.
The β-isomeric [VIV14Sb8O42]4− polyoxoanion is a component of the solvothermally prepared ammonium salt (NH4)4[V14Sb8O42]·2H2O (Table 4).185 The structure is further characterised by comparably short intercluster Sb⋯O contacts of 2.83–2.98 Å.
{V15Sb6}-type polyoxoanion. The compound (H3tren)2[V15Sb6O42]·0.33(tren)·nH2O (n = 3–5) was obtained under solvothermal conditions (Table 4),186 comprising an isostructural antimony analogue [VIV15Sb6O42]6− of the molecular magnet [H2O@VIV15As6O42]6−. The nanosized structure of this “fully-reduced” polyoxoanion (Fig. 25a, left) is viewed as a derivative of the {V18O42} archetype where three {VO5} square-pyramids are substituted by three handle-like {Sb2O5} groups. The structure features {V15}-nuclearity building bocks being arranged in hexagonal layers via weak, intercluster Δ(Sb–μ-O⋯Sb–μ-O⋯Sb–μ-O) interactions within the distances of ca. 2.9 Å, thus resulting in the formation of trimeric super-structures ([VIV15Sb6O42]6−)3. The spin-1/2 ground structure of the [VIV15Sb6O42]6− polyoxoanion “represents an interesting reference system for the concise characterisation of the microscopic magnetism of these spin 1/2 triangle systems”186 (Fig. 25a, right).
{V16Sb4}-type polyoxoanion. The above class of SbPOV-based compounds with the general formula [(Hamine)mV18−zSb2zO42]·nH2O (z = 2–4) was extended by a new member of this series, namely (H2aep)4[V16Sb4O42]·2H2O, prepared under solvothermal conditions (Table 4).185 This compound is composed of the “fully-reduced” [VIV16Sb4O42]8− polyoxoanion (D2h symmetry) charge-balanced by four doubly protonated (C6H17N3)2+ groups (=H2aep). The structure of the SbPOV showing the common rhombicuboctahedral topology consists of sixteen {VO5} square pyramids and four {SbO3} trigonal pyramids and can be interpreted as being derived from the {V18O42} archetype when replacing two {VO5} units in the latter with two {Sb2O5} groups. In the structure of (H2aep)4[V16Sb4O42]·2H2O, the SbPOVs are connected into infinite chains via intercluster Sb⋯O interactions of ca. 2.85 Å (Fig. 25b).
Zinc–SbPOV hybrids. Two organic–inorganic hybrid solids [Zn2(dien)3][{Zn(dien)}2V16Sb4O42(H2O)]·4H2O and [Zn(dien)2]2[{Zn(dien)}2(V14Sb8O42)2(H2O)]·4H2O were obtained by pH-controlled hydrothermal syntheses (Table 4).189 These compounds contain the [VIV16Sb4O42]8− and β-[VIV14Sb8O42]4− building blocks and in the solid state are arranged as 1D infinite linear and zig-zag chains, respectively. In the structure of the former compound, neighbouring [H2O@V16Sb4O42]8− polyoxoanions with D2h symmetry are linked by the [Zn(dien)]2+ bridging groups via covalent Zn–O bonds (Fig. 28a), while the [Zn2(dien)3]4+ complexes resemble structurally discrete countercations. Similarly to the previous compound, the crystal structure of [Zn(dien)2]2[{Zn(dien)}2(V14Sb8O42)2(H2O)]·4H2O contains neighbouring β-[VIV14Sb8O42]4− polyoxoanions that are bridged by single [Zn(dien)]2+ moieties through covalent Zn–O bonds (Fig. 28b). The two [Zn(dien)2]2+ cations compensate the negative charge of the [{Zn(dien)}2(V14Sb8O42)2(H2O)]4− assembly.
A [H2O@VIV16Sb4O42]8− polyoxoanion in which the geometric position of the handle-like {Sb2O5} groups reduce the symmetry of the {V16} skeleton to C2 (vs. D2h symmetry reported for all hitherto characterised {V16}-nuclearity AsPOVs and SbPOVs) was also identified (Fig. 29).190 For comparison, the [VIV16Si4O46]12− polyoxoanion illustrated in Fig. 6a has D2h symmetry, whereas the [VIV16Ge4O42(OH)4]8− in Fig. 8a also displays C2 symmetry. Such changes in the SbPOV structure influenced the geometrical parameters, specifically the V⋯V distances of this polyoxoanion. While the shortest non-bonding V⋯V distances in the C2-symmetrical [H2O@V16Sb4O42]8− structure are between 2.73 and 3.10 Å, those in the isonuclear, but D2h-symmetrical analogues fall in the range 2.85–3.06 Å in (H2aep)4[V16Sb4O42]·2H2O,185 2.92–3.03 Å in [V16Sb4O42(H2O){VO(dach)2}4]·(dach)·10H2O,187 and 2.92–3.05 Å in [Zn2(dien)3][{Zn(dien)}2V16Sb4O42(H2O)]·4H2O.189 The “fully-reduced” [H2O@VIV16Sb4O42]8− polyoxoanion with its new pseudorhombicuboctahedral topology is a component of the [Zn(tren)(H2tren)]2[V16Sb4O42(H2O)]·nH2O compound (n = 6–10) obtained under solvothermal conditions (Table 4). Two trigonal bipyramidal {Zn(tren)(H2tren)}4+ complexes assume the role of countercations. The crystal structure of this compound that is soluble in methanol and ethanol contains channels between the SbPOVs arranged in the pairs by weak intercluster Sb⋯O interactions because of the specific orientation of the handle-like [Sb2O5]4− groups. The intermolecular N–H⋯O hydrogen bonds between the cations, polyoxoanions and H2O molecules expand the structure of [Zn(tren)(H2tren)]2[V16Sb4O42(H2O)]·nH2O into a 3D network. The low-field magnetic susceptibility studies revealed that the C2-symmetrical [H2O@VIV16Sb4O42]8− polyoxoanion exhibits strong antiferromagnetic coupling between the spin-1/2 vanadyl {VO}2+ moieties. Remarkably, a significant difference in the susceptibility data was found between the compounds [Zn(tren)(H2tren)]2[V16Sb4O42(H2O)]·nH2O (with C2-symmetrical {V16} spin polytope) and [Zn2(dien)3][{Zn(dien)}2V16As4O42(H2O)]·3H2O159 (with D2h-symmetrical {V16} spin polytope), thus showing the effect of the pnictogen oxide positions on the magnetism of the isonuclear, but not isostructural AsPOV and SbPOV structures.
Cobalt– and nickel–SbPOV hybrids. The compound [{Co(en)2}2V14Sb8O42(H2O)]·6H2O prepared under hydrothermal conditions (Table 4) was the first example of functionalisation of a POV building block with antimony.191 The crystal structure features 2D arrays of the spherical β-[H2O@VIV14Sb8O42]4− polyoxoanions that are joined through the covalent bonds between the apical oxygen atoms of the β-SbPOVs and the bridging Co2+ ions of the [Co(en)2]2+ complexes (Fig. 28c). Similar to the β-[VIV14As8O42]4− polyoxoanion (Fig. 15b), this “fully-reduced” SbPOV represents a modified [V18O42]12− structure whose four {VO5} square pyramids are replaced by four handle-like {Sb2O5} groups.
The compounds [Co(tren)(H2O)]3[V15Sb6O42(H2O)]·H2O, [Co2(tren)3]2[Co(tren)(en)][{V15Sb6O42(H2O)(Co(tren)2)}V15Sb6O42(H2O)]·nH2O (n ≈ 11), and [Co(tren)(H2tren)]2[V16Sb4O42(H2O)]·6H2O containing distinct Co2+ complexes, namely [Co(tren)(H2O)]2+, [Co2(tren)3]4+, [Co(tren)(en)]2+, [Co(tren)2]2+, and [Co(tren)(H2tren)]4+ were synthesised under solvothermal conditions using different concentrations of amine molecules (Table 4).192 Notably, the solvothermal reaction yielding [Co2(tren)3]2[Co(tren)(en)][{V15Sb6O42(H2O)(Co(tren)2)}V15Sb6O42(H2O)]·nH2O included a partial decomposition of tren molecules yielding bidentate en ligands. The inorganic–organic hybrid solids presented above are based on the [H2O@VIV15Sb6O42]6− or [H2O@VIV16Sb4O42]8− polyoxoanions with diameters of ca. 11 Å. The “fully-reduced” SbPOV shells with the shortest V⋯V distances of 2.81–3.07 Å are derived from the {V18O42} shell showing D4d symmetry, i.e. 3 {VO5} ↔ 3 {Sb2O5}. The [H2O@VIV15Sb6O42]6− polyoxoanion in the crystal structure of [Co(tren)(H2O)]3[V15Sb6O42(H2O)]·H2O are involved in weak Sb⋯O intercluster interactions, with the shortest one being ca. 2.90 Å. These Sb⋯O interactions are comparable with those identified e.g., in the 2D layered structures of the compounds (H2en)2[VIV14Sb8O42(H2O)]·3H2O (dSb⋯O = 2.72–2.92 Å) and (H2ppz)2[VIV14Sb8O42(H2O)] (dSb⋯O = 2.83–3.01 Å) with the discrete β-isomeric SbPOV motifs (dV⋯V = 2.90– 3.10 Å).193 The solvothermal formation of these compounds is accompanied by a decomposition of N,N,N′,N′-tetramethylethylenediamine to ethylenediamine and in situ fragmentation of 1-methylpiperazine to piperazine, respectively. Like for the compounds (H2en)2[V14Sb8O42(H2O)]·3H2O and (H2ppz)2[V14Sb8O42(H2O)], OSbPOV⋯Hamine hydrogen bonds are observed in the crystal structure of [Co(tren)(H2O)]3[V15Sb6O42(H2O)]·H2O. The crystal structure of [Co2(tren)3]2[Co(tren)(en)][{V15Sb6O42(H2O)(Co(tren)2)}V15Sb6O42(H2O)]·nH2O (n ≈ 11) is characterised by short Sb⋯N interactions (2.60 Å, 2.65 Å, and 2.74 Å), which engage the N atoms of primary amine molecules (tren) and the Sb atoms of the SbPOVs. Relatively short Sb⋯O intercluster contacts (2.88–2.93 Å) were also observed. The observed N–H⋯O hydrogen bonds generate a 3D network. Interestingly, the central [H2O@VIV16Sb4O42]8− polyanion of the compound [Co(tren)(H2tren)]2[V16Sb4O42(H2O)]·6H2O is isostructural to that of [Zn(tren)(H2tren)]2[V16Sb4O42(H2O)]·nH2O (n = 6–10)190 (see Fig. 29) and can be viewed as being derived from the Td-symmetric {V18O42} shell, i.e. by formal metathesis 2{VO5} ↔ 2{Sb2O5}. The shortest V⋯V distances in this C2-symmetrical SbPOV polyoxoanion are in the range 2.73–3.09 Å.
The compounds [Co(aepda)2]2[{Co(aepda)2}V15Sb6O42(H2O)]·5H2O and [Ni(aepda)2]2[{Ni(aepda)2}V15Sb6O42(H2O)]·8H2O (aepda = N-(2-aminoethyl)-1,3-propanediamine = C5H15N3) were also obtained under solvothermal conditions194 (Table 4) and their crystal structures feature the [H2O@VIV15Sb6O42]6− polyoxoanion being structurally related to the well-known {V18O42} archetype, as is the case for the AsPOV analogue [H2O@VIV15As6O42]6−. Each of these [H2O@VIV15Sb6O42]6− polyoxoanions comprises a terminal oxygen position covalently bonded to the transition metal complexes [M(aepda)2]2+ (M = Co, Ni). As a result, the M2+ centre has an octahedral M(Naepda)5OSbPOV coordination environment. The two remaining [M(aepda)2]2+ moieties in each compound serve as charge-balancing cations. As in the case of several other TMC-functionalised heteroPOVs, the paramagnetic M2+ ions (M = Co, Ni) from the terminally coordinated [M(aepda)2]2+ complexes couple only very weakly with the spin-1/2 vanadyl {VO}2+ moieties of the [H2O@VIV15Sb6O42]6− polyoxoanions. In the crystal structure of the compound comprising Co2+ ions, the polyoxoanions are connected through weak intercluster Sb⋯O (3.13 Å) interaction, thus forming pairs of SbPOVs (Fig. 28d). In contrast, in the crystal structure of the compound containing Ni2+ ions pairs of SbPOVs linked by weak Sb⋯N contacts (2.65 Å) are observed. These Sb⋯N interactions involve the Sb atoms of the “fully-reduced” [H2O@VIV15Sb6O42]6− polyoxoanion and the N atoms of the propylamine chains from the adjoined [Ni(aepda)2]2+ fragments (Fig. 28e). In addition, intermolecular N–H⋯O hydrogen bonds generating higher dimensional networks were observed in the structures of both these compounds.
A dimeric {[Ni2(tren)3(V15Sb6O42(H2O)0.5)]2}4− fragment where two “fully-reduced” [VIV15Sb6O42(H2O)0.5]6− polyoxoanions (shortest dV⋯V = 2.84–3.08 Å) are bridged by an in situ-produced [Ni2(tren)3]4+ complex via a multidentate μ-1,7 tren ligand was identified in the solvothermally synthesised, highly insoluble compound [Ni(Htren)2][Ni2(tren)3(V15Sb6O42(H2O)0.5)]2·H2O (Table 4).195 Its crystal structure is characterised by double chains along the crystallographic b axis, formed by short Sb⋯N contacts (dSb⋯N = ca. 2.69 Å) between the neighbouring heterometallic dimers. Again, an additional [Ni(Htren)2]4+ complex with protonated tren ligands acts as countercation. For comparison, the covalent Sb–N distances in the structures of the aforementioned compounds [V14Sb8(Haep)4O42(H2O)]·4H2O and (H2aep)2[V15Sb6(Haep)2O42(H2O)]·2.5H2O (Fig. 27) are dSb–N = 2.53 and 2.54 Å and dSb–N = 2.50 and 2.54 Å, respectively.188 A 3D network in the crystal structure of [Ni2(tren)3(V15Sb6O42(H2O)0.5)]2[Ni(Htren)2]·H2O is generated by a complex hydrogen bonding pattern between H atoms of water molecules and the amine moieties and oxygen atoms of the SbPOVs.
Two pseudopolymorphic compounds with compositions [Ni(dien)2]3[V15Sb6O42(H2O)]·nH2O (n = 12 and 8) and the compound [Ni(dien)2]4[V16Sb4O42(H2O)] were prepared solvothermally by adjusting the reaction temperature (Table 4).196 The pseudopolymorphs display the spherical [H2O@VIV15Sb6O42]6− polyoxoanions which are virtually isostructural to the molecular magnet [H2O@VIV15As6O42]6−. The structural motif of the “fully-reduced” [VIV15Sb6O42]6− polyoxoanion is shown in Fig. 25a and that of [VIV16Sb4O42]8−, a component of the compound [Ni(dien)2]4[V16Sb4O42(H2O)], is illustrated in Fig. 26. Interestingly, the crystal structures of the pseudopolymorphs possess different composition of the asymmetric units that involve four crystallographically independent [H2O@VIV15Sb6O42]6− polyoxoanions and twelve [Ni(dien)2]2+ complexes adopting the s-fac, mer-, and u-fac-configurations (tetragonal space group) or a third of the polyoxoanion and a mer-{Ni(dien)2}2+ complex (rhombohedral space group). In the crystal structure of the compound crystallising in the tetragonal space group, the SbPOVs are arranged as tetrameric superstructures with composition ([H2O@VIV15Sb6O42]6−)4 (layer-like arrangement). The individual polyoxoanions are held together by weak Sb⋯O interactions (2.68 to 2.97 Å), which are significantly shorter than the sum of van der Waals radii (3.52 Å) for Sb and O atoms. For the structure crystallising in the rhombohedral space group, the shortest intercluster Sb⋯O distances exceed 5 Å, because of a triangular-like arrangement of dicationic mer-[Ni(dien)2]2+ complexes around the [VIV15Sb6O42]6− polyoxoanions. The 3D network structure of this compound is realised by intermolecular N–H⋯O hydrogen bonding interactions. The compound [Ni(dien)2]4[V16Sb4O42(H2O)] comprises octahedrally coordinated Ni2+ ions in the [Ni(dien)2]2+ cations adopting the s-fac- and u-fac-configurations. In the crystal structure of [Ni(dien)2]4[V16Sb4O42(H2O)], the [H2O@VIV16Sb4O42]8− polyoxoanions are arranged in layers in the (100) plane.
Iron–SbPOV hybrids. The solvothermally synthesised compound [{Fe(dach)2}3{V15Sb6O42(H2O)}]·8H2O with the unique porous 3D network topology in its extended solid-state structure (Fig. 30) is the first example of an iron-augmented SbPOV architecture also exhibiting a unique magnetic structure.197 The amine molecules used in the synthesis of this compound (Table 4) were shown to carry out versatile functions, acting as ligands, solvents, and reducing agents. In the structure, the [H2O@VIV15Sb6O42]6− polyoxoanion is expanded by six in situ-generated [Fe(dach)2]2+ complexes, which coordinate terminally to the polyoxoanion via Fe–O bonds to form octahedral {FeN4O2} units that further interlink the adjoined SbPOVs. Relatively strong N–H⋯O hydrogen bonds were observed. The compound is further characterised by an optical energy gap of 2.47 eV. The low-field magnetic susceptibility revealed ferromagnetic exchange interactions between the spin-1/2 vanadyl {VO}2+ moieties of the polyoxoanion and the high-spin (S = 2) Fe2+ ions of the adjoined [Fe(dach)2]2+ complexes. We note that this heterometal-vanadyl coupling differs significantly from that established for the aforementioned TMC-supported AsPOVs and SbPOVs, which showed only weak or negligible, and mostly antiferromagnetic interactions between the spin centres of the central building blocks and the attached TMC fragments.
The study of the chemical and physical properties and reactivity of the SbPOVs has been motivated, in particular, by the following points closely related to catalysis:
(i) The SbIII ions exhibited stabilising effects on the Keggin-type POM structures at high temperatures (>450 °C) and this played an important role in employing the Sb-modified POMs as catalysts, e.g. for the oxidative dehydrogenation of ethane to ethylene.198
(ii) Since Sb-modified vanadium catalysts were shown to selectively oxidise o-xylene to phthalic anhydride,199 SbPOVs might find potential applications as pre- or catalysts in heterogeneous selective oxidation reactions.
4. Summary and outlook
We reviewed the structural aspects and key properties of heavier group 14 and 15 element-functionalised POVs and their inorganic–organic hybrid compounds involving transition metal and lanthanoid complexes as charge compensating units and structure modifiers. We provided an overview of the synthetic routes to SiPOVs, GePOVs, AsPOVs and SbPOVs whose central structural motifs typically are derived from the {V18O42} archetype and display rhombicuboctahedral topologies. The V4+ cations in these robust polyoxoanions incorporating handle-like {E2O7}/{E2O5S2} (E = Si or Ge) and {E2O5} (E = As or Sb) groups adopt the very common square-pyramidal {VO5} coordination geometry, although a number of compounds involving {VO6} octahedra and {VO4} tetrahedra were also reported. Note that the crystal chemical aspects of vanadium oxide polyhedra were comprehensively analysed by Schindler et al., in 2000200 and were, therefore, not discussed herein. The topological aspects of POV macropolyhedra can be found in the work of King dating back to 1995.201It was shown that the {Ge2O5S2} and {As2O5}/{Sb2O5} substituents in the {V15E6}-type building blocks play crucial roles in superexchange interactions within the central spin-1/2 {V3} triangle that define the low-temperature magnetism of these heteroPOVs. Even though the [H2O@V15Ge6O42S6]12−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102 and [V15Sb6O42]6−
102 and [V15Sb6O42]6−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 polyoxoanions feature spin topologies very similar to that of the prominent molecular magnet [H2O@V15As6O42]6−,151 the magnetic exchange properties of the {Ge2O5S2}-modified POVs differed significantly from those of the corresponding As- and Sb-functionalised POVs.
186 polyoxoanions feature spin topologies very similar to that of the prominent molecular magnet [H2O@V15As6O42]6−,151 the magnetic exchange properties of the {Ge2O5S2}-modified POVs differed significantly from those of the corresponding As- and Sb-functionalised POVs.
As was demonstrated by a large number of studies, the discrete heteroPOVs can be extended further towards the hybrid inorganic–organic frameworks by introducing TMCs or lanthanoid salts to the reactions mixtures. These TMCs and lanthanoid complex cations commonly act as bridging ligands between the neighbouring heteroPOVs and often exhibit unusual coordination geometries and connectivities. The transition metal ions (Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+) can be introduced into the backbone structures of the vanadium oxide shells to facilitate the linking of these heteroPOVs into 1D, 2D and 3D networks and to enrich/modify their magnetic properties. We did not highlight the structural chemistry of TMC fragments in these structures; discussion on their coordination geometries and bonding patterns can be found in the original literature. The use of adjoined inorganic moieties (e.g. lanthanoid complex cations150) was shown to result in high coordination numbers (up to 6) for the POV shells and, thus, to remarkably increase the overall thermal stabilities of these heteroPOV-based materials (even exceeding 500 °C). The transition metal functionalisation of the polynuclear molecular vanadium oxides open up new possibilities for making new heteroPOV spin architectures with e.g. spin-glass behaviour by virtue of the fact that the vanadyl {VO}2+ moieties in these polyoxoanions can be replaced by divalent transition metal ions.
Another way to improve the thermal stability of heteroPOVs may be through the preparation of heteroPOV-based ionic liquids, which can be achieved by pairing negatively charged heteroPOV building blocks with appropriate cations such as imidazolium, pyridinium, phosphonium species. For instance, a number of Keggin and Lindqvist polyoxomolybdate and polyoxotungstate-based ionic liquids202 and tetraalkylphosphonium decavanadates203 have been reported.
The reactivity of heteroPOVs towards organic groups was also illustrated. The organic amines usually introduced in the reaction mixtures are commonly not only acting as reducing agents, but also as structure-directing and charge-compensating groups and, furthermore, display interesting transformations under hydro-/solvothermal conditions in the presence of VV sources. The organic functionalisation of magnetic polyoxoanions, as exemplified by [H2O@VIV14Sb8(Haep)4O42] and [H2O@VIV15Sb6(Haep)2O42]4−,188 enabled their dissolution in polar protic solvents (methanol, ethanol, etc.). It also can partly or fully compensate the high negative charges of the heteroPOVs, an important issue for molecular deposition and subsequent (micro)spectroscopic studies with this class of compounds. Such a direct covalent attachment of organic ligands allows for the expansion of heteroPOVs into classical coordination clusters and may significantly influence their redox and acid–base properties.204
The relevance of the presented polyoxoanions to different fields of chemical and physical sciences was briefly discussed. The heteroPOVs are envisaged to find potential applications in molecular electronics and spintronics, optics, sorption, catalysis and artificial photosynthesis.205 However, the search for heteroPOV compounds with sufficient solubility in organic solvents and a high structural and thermal stability is crucial in this context. The synthesis of new discrete and multidimensional SiPOVs, GePOVs, AsPOVs and SbPOVs is highly desirable in order to discover strategies for altering their chemical and physical properties.
Since the ratio of VV/VIV and VIV/VIII redox couples and molecular connectivities are strongly influenced by the nature of the heteroelements, and since the overall E/VV/VIV/VIII ratio impacts the overall properties of the heteroPOV to a large extent, the chemical modification of the conventional POV shells by heavier metal ions from groups 14 such as Sn and Pb is appealing and worthwhile. For example, functionalisation of POVs with bismuth, the heaviest group-15 element, resulted in interesting photocatalytic activity of the designed molecular bismuth vanadium oxide clusters.206 The reactivities of SiPOVs, GePOVs, AsPOVs and SbPOVs towards transition metals e.g. from the platinum group and actinoids are yet to be discovered. Integration of lanthanoid ions into heteroPOV shells may lead to interesting magnetic phenomena as well as visible-light photocatalytic properties. The controlled interlinking of heteroPOV spin structures is of high importance for the development of smart materials with electron storage functions and inter-site communications. The coexistence of (partly) delocalised 3d-vanadium electron density with localised spins of transition metals and lanthanoids in the mixed-valent heteroPOV-based inorganic–organic hybrid materials may result in unusual spintronic effects, “in particular, if the corresponding charge transport can be confined to one or two dimensions”.114
The molecular magnetism of heteroPOVs described so far is characterised by the presence of strong antiferromagnetic exchange interactions between the vanadium spin centres, with the exception of several compounds showing ferrimagnetic ordering characteristics. Because there are some indications of ferromagnetic coupling interactions in heteroPOVs (see the mixed-valent [HCO2@VIV6VV6As8O40]3− polyoxoanion119,120) as well as alkoxoPOVs (see the mixed-valent [VIV4VV2O7(OR)12] compound78 and the mixed-valent [VIIIVIV5O6(OMe)8(calix)(MeOH)]− polyoxoanion207 where calix = p-tert-butylcalix[4]arene), the magnetic properties of these compounds should be explored in more detail. Building a deeper understanding of the magnetism of the mixed-valent, “fully-reduced” and “highly-reduced” SiPOVs, GePOVs, AsPOVs and SbPOVs, which can potentially act as “nanoscale quantum magnets” and spin qubits remains challenging, because of the absence of magnetochemical models suitable for the fitting of their magnetic susceptibility data.
Only a few studies have employed quantum chemical models to study the electronic structures of fully-oxidised POVs208 and of some of the herein-described “fully-reduced”, semimetal-functionalised POVs with the V14 and V15 nuclearities.209 Reliable computational methods for assessing the electronic and magnetic properties of the mixed-valent heteroPOVs are still being developed.
The biological and medicinal relevance of POVs210 deserves mention, as the archetypal decavanadate [V10O28]6− has demonstrated non-trivial chemical behaviour and reactivity towards biomolecules,211e.g.: (i) inhibition of various enzymes,212 (ii) hydrolytic DNA cleavage,213 (iii) inhibition of oxygen consumption in membranes.214 The interactions of other, lower nuclearity, but more labile oxovanadates ([VO4]3− as a structural and electronic analogue of phosphate, [V2O7]4−, and [V4O12]4−) with enzymes215 have also been recognised and well-documented.1,216 Over the past two decades, much attention has been paid to biochemical, cell biological, antidiabetic and antitumor studies involving vanadium217–219 and organic biomolecules for the reason that “understanding the nature of protein interactions with oxovanadates and other oxometalates is important for further development of drugs based on vanadium complexes and polyoxoanions”.1 In view of the biological innocuity of bismuth and its extensive uses in medicine, pharmaceuticals, and cosmetics, the class of polyoxovanadatobismuthates is relevant to biochemical studies.
Although the number of reports on magnetic heteroPOVs has grown substantially in the last few decades, the field is hampered by the lack of systematic research on this class of molecular compounds. Many questions concerning their inorganic synthesis, reactivity in aqueous and non-aqueous solutions, molecular magnetism, spectroscopy (in particular, NMR studies in solution and in the solid state), excited states and computational application remain entirely open. The benefits of effective adjustment of the heteroPOV building block's properties from the standpoint of chemical reactivity, electrochemistry, molecular magnetism, photophysics and surface physics will be remarkable.
Finally, we note that the self-assembly formation mechanisms of heteroPOVs remain poorly understood and for the development of rational syntheses these mechanisms should be unveiled. There are first promising attempts in this direction: in situ energy-dispersive X-ray diffraction experiments were performed under hydrothermal conditions in order to gain insight into the synthetic parameters influencing the formation of SbPOVs.220 These studies were able to demonstrate directly that e.g. the amine concentration plays a crucial role for the crystallisation of a distinct SbPOV, where the formation of [V14Sb8(Haep)4O42(H2O)]·4H2O188 is observed at the lowest amine concentration, whereas (H2aep)2[V15Sb6(Haep)2O42(H2O)]·2.5H2O188 crystallised at slightly larger amine concentration and finally the SbPOV with the highest number of VIV centres, (H2aep)4[V16Sb4O42]·2H2O,185 was obtained at the highest amine concentration. In addition, solvothermal syntheses performed under static conditions yield mixtures of these three SbPOVs while stirring afforded formation of phase pure materials at a distinct amount of amine in the reaction slurries. These empirical in situ studies pave the way for a more comprehensive understanding of the complex self-assembly processes in vanadate reaction solutions, in line with similarly revealing in situ spectroscopy studies in the chemistry of other polyoxometalate families.221
Abbreviations
Methods
| CV | Cyclic voltammetry |
| DFT | Density functional theory |
| EA | Elemental analysis |
| EDXS | Energy-dispersive X-ray spectroscopy |
| EMP | Electron microprobe |
| EPR | Electron paramagnetic resonance |
| ESI-MS | Electrospray ionisation mass spectrometry |
| INS | Inelastic neutron scattering |
| IR | Infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| powder XRD | Powder X-ray diffraction |
| SEM | Scanning electron micrograph |
| single-crystal XRD | Single-crystal X-ray diffraction |
| TGA | Thermogravimetric analysis |
| UV-vis | Ultraviolet-visible spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
Inorganic compounds
| POV | Polyoxovanadate |
| heteroPOV | Heteropolyoxovanadate |
| alkoxoGePOV | Polyoxoalkoxovanadatogermanate |
| AsPOV | Polyoxovanadatoarsenate |
| GePOV | Polyoxovanadatogermanate |
| SiPOV | Polyoxovanadatosilicate |
| SbPOV | Polyoxovanadatoantimonate |
| TMC | Transition metal complex |
Organic compounds
| 2,2′-bipy | 2,2′-Bipyridine |
| aep | 1-(2-Aminoethyl)piperazine |
| aepda | N-(2-Aminoethyl)-1,3-propanediamine |
| bbi | 1,1′-(1,4-Butanediyl)bis(imidazole) |
| bpe | 1,2-Bis(4-pyridyl)ethylene |
| dab | 1,4-Diaminobutane |
| dach | (±)-trans-1,2-Cyclohexanediamine |
| dien | Diethylenetriamine |
| en | Ethylenediamine |
| enMe | 1,2-Diaminopropane |
| pdn | 1,3-Propanediamine |
| phen | 1,10-Phenanthroline |
| ppz | Piperazine |
| salen | N,N′-(Ethylene)bis(salicylideneiminate) |
| teos | Tetraethyl orthosilicate |
| tepa | Tetraethylenepentamine |
| theed | N,N,N′,N′-Tetrakis(2-hydroxyethyl)ethylenediamine |
| tren | Tris(2-aminoethyl)amine |
Acknowledgements
K.Y.M. is grateful to the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral reintegration fellowship and the Jülich-Aachen Research Alliance – Future Information Technology (JARA-FIT) for a Seed Fund grant. We thank Dr Claire Besson, Dr Jeff Rawson (RWTH Aachen University) and Dr Natalya Izarova (Forschungszentrum Jülich) for helpful suggestions.References
- D. C. Crans, Comments Inorg. Chem., 1994, 16, 35 CrossRef CAS and references cited therein.
- (a) N. D. Chasteen, in Vanadium in Biological Systems: Physiology and Biochemistry, ed. N. D. Chasteen, Kluwer Academic Publishers, Boston, 1990 Search PubMed; (b) D. Rehder, Angew. Chem., Int. Ed. Engl., 1991, 30, 148 CrossRef; (c) D. C. Crans, C. M. Simone and J. S. Blanchard, J. Am. Chem. Soc., 1992, 114, 4926 CrossRef CAS; (d) A. Butler and C. J. Carrano, Coord. Chem. Rev., 1991, 109, 61 CrossRef CAS.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, Wiley Interscience, New York, 1999, 6th edn, p. 714 Search PubMed.
- A. S. Tracey, G. R. Willsky and E. S. Takeuchi, Vanadium: Chemistry, Biochemistry, Pharmacology and Practical Applications, CRC Press, Boca Raton, 2007 Search PubMed.
- (a) R. D. Srivastava, Heterogeneous Catalytic Science, CRC Press, Boca Raton, 1988 Search PubMed; (b) G. Busca and G. Centi, J. Am. Chem. Soc., 1989, 111, 46 CrossRef CAS; (c) G. Deo, F. D. Hardcastle, M. Richards, A. M. Hirt and I. E. Wachs, in Novel Materials in Heterogeneous Catalysis, ed. R. T. K. Baker and L. L. Murrell, American Chemical Society, Washington, D.C., 1990 Search PubMed; (d) D. Patel, P. J. Anderson and H. H. Kung, J. Catal., 1990, 125, 132 CrossRef CAS; (e) F.-L. Wang and W.-S. Lee, J. Chem. Soc., Chem. Commun., 1991, 1760 RSC; (f) B. I. Whittington and J. R. Anderson, J. Phys. Chem., 1993, 97, 1032 CrossRef CAS; (g) P. Dinka, K. Prandová and M. Hronec, Appl. Clay Sci., 1998, 13, 467 CrossRef CAS; (h) A. G. J. Ligtenbarg, R. Hage and B. L. Feringa, Coord. Chem. Rev., 2003, 237, 89 CrossRef CAS; (i) J. Pless, B. Bardin, H.-S. Kim, D. Ko, M. Smith, R. Hammond, P. Stair and K. Poeppelmeier, J. Catal., 2004, 223, 419 CrossRef CAS; (j) A. R. Gaspar, D. V. Evtuguin and C. P. Neto, Ind. Eng. Chem. Res., 2004, 43, 7754 CrossRef CAS; (k) J. D. Pless, D. Ko, R. R. Hammond, B. B. Bardin, P. C. Stair and K. R. Poeppelmeier, J. Catal., 2004, 223, 419 CrossRef CAS; (l) Z. Strassberger, E. V. Ramos-Fernandez, A. Boonstra, R. Jorna, S. Tanase and G. Rothenberg, Dalton Trans., 2013, 42, 5546 RSC.
- (a) P. Csermely, A. Martonosi, G. C. Levy and A. J. Ejchart, Biochem. J., 1985, 230, 807 CrossRef CAS; (b) D. C. Crans and S. M. Schelble, Biochemistry, 1990, 29, 6697 CrossRef; (c) D. C. Crans and C. M. Simone, Biochemistry, 1991, 30, 6734 CrossRef CAS; (d) See also a special issue of Coord. Chem. Rev., 2003, 237, 3.
- (a) J. Livage, Chem. Mater., 1991, 3, 578 CrossRef CAS; (b) C. J. Brinker and G. W. Scherer, Sol-Gel Science, Academic Press, New York, 1991 Search PubMed; (c) J. Livage, Solid State Ionics, 1996, 86–88, 935 CrossRef CAS . See, also: ; (d) F. J. Anaissi, G. J. F. Demets, H. E. Toma and A. C. V. Coelho, J. Electroanal. Chem., 1999, 464, 48 CrossRef CAS.
- A. R. Raju and C. N. R. Rao, J. Chem. Soc., Chem. Commun., 1991, 1260 RSC.
- (a) H. T. Evans Jr. and S. Landergren, in Handbook of Geochemistry, ed. K. H. Wedepohl, Springer, Berlin, ch. 23, vol. II/2, 1978 Search PubMed; (b) H. T. Evans Jr. and J. S. White Jr., Mineral. Rec., 1987, 18, 333 Search PubMed.
- J. Do and A. J. Jacobson, Inorg. Chem., 2001, 40, 2468 CrossRef CAS.
- (a) M. G. Kanatzidis, C.-G. Wu, H. O. Marcy and C. R. Kannewurf, J. Am. Chem. Soc., 1989, 111, 4139 CrossRef CAS; (b) M. Z. A. Munshi, W. H. Smyrl and C. Schmidtke, Chem. Mater., 1990, 2, 530 CrossRef CAS; (c) J.-M. Amarilla, B. Casal, J.-C. Galvan and E. Ruiz-Hitzky, Chem. Mater., 1992, 4, 62 CrossRef CAS.
- (a) G. C. Bond and S. F. Tahir, Appl. Catal., 1991, 71, 1 CrossRef CAS; (b) M. S. El-Shall, W. Slack, W. Vann, D. Kane and D. Hanley, J. Phys. Chem., 1994, 98, 3067 CrossRef CAS; (c) S. Surnev, M. G. Ramsey and F. P. Netzer, Prog. Surf. Sci., 2003, 73, 117 CrossRef CAS; (d) R. Schlögl and S. B. Abd Hamid, Angew. Chem., Int. Ed., 2004, 43, 1628 CrossRef.
- (a) C. R. Walk and J. S. Gore, J. Electrochem. Soc., 1975, 122, 68C Search PubMed; (b) M. S. Whittingham, J. Electrochem. Soc., 1976, 123, 315 CrossRef CAS; (c) M. S. Whittingham, R. Chen, T. Chirayil and P. Y. Zavalij, Proc. - Electrochem. Soc., 1996, 95–96, 76 Search PubMed; (d) P. Gomez-Romero, Adv. Mater., 2001, 13, 163 CrossRef CAS; (e) A. Bose, P. He, C. Liu, B. D. Ellman, R. J. Twieg and S. D. Huang, J. Am. Chem. Soc., 2002, 124, 4 CrossRef CAS; (f) L. Chen, F. Jiang, Z. Lin, Y. Zhou, C. Yue and M. Hong, J. Am. Chem. Soc., 2005, 127, 8588 CrossRef CAS; (g) L. Li, S. Kim, W. Wang, M. Vijayakumar, Z. Nie, B. Chen, J. Zhang, G. Xia, J. Hu, G. Graff, J. Liu and Z. Yang, Adv. Energy Mater., 2011, 1, 394 CrossRef CAS; (h) S. Uematsu, Z. Quan, Y. Suganuma and N. Sonoyama, J. Power Sources, 2012, 217, 13 CrossRef CAS.
- See, e.g. H.-U. Meisch and L. J. M. Becker, Biochim. Biophys. Acta, Bioenerg., 1981, 636, 119 CrossRef CAS.
- (a) J. Livage, Coord. Chem. Rev., 1998, 178–180, 999 CrossRef CAS; (b) Y. Hayashi, Coord. Chem. Rev., 2011, 255, 2270 CrossRef CAS.
- (a) M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef; (b) L. Cronin, P. Kögerler and A. Müller, J. Solid State Chem., 2000, 152, 57 CrossRef CAS; (c) A. Müller, P. Kögerler and H. Bögge, Struct. Bonding, 2000, 96, 203 CrossRef; (d) D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS.
- See for example (a) B. Dong, J. Peng, A. Tian, J. Sha, L. Li and H. Liu, Electrochim. Acta, 2007, 52, 3804 CrossRef CAS; (b) T. D. Keene, D. M. D'Alessandro, K. W. Krämer, J. R. Price, D. J. Price, S. Decurtins and C. J. Kepert, Inorg. Chem., 2012, 51, 9192 CrossRef CAS and references cited therein.
- (a) L. Chen, F. Jiang, Z. Lin, Y. Zhou, C. Yue and M. Hong, J. Am. Chem. Soc., 2005, 127, 8588 CrossRef CAS . See also: ; (b) Q. Chen and J. Zubieta, Coord. Chem. Rev., 1992, 114, 107 CrossRef CAS.
- W. G. Klemperer, T. A. Marquart and O. M. Yaghi, Angew. Chem., Int. Ed. Engl., 1992, 31, 49 CrossRef.
- G. Huan, A. J. Jacobson and V. W. Day, Angew. Chem., Int. Ed. Engl., 1991, 30, 422 CrossRef.
- (a) F. J. Rossotti and H. Rossotti, Acta Chem. Scand., 1956, 10, 957 CrossRef CAS; (b) P. A. Durif and M. T. Averbuch-Pouchot, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 680 CrossRef; (c) A. S. J. Wery, J. M. Gutierrez-Zorrila, A. Luque, P. Roman and M. Martinez-Ripoll, Polyhedron, 1996, 15, 4555 CrossRef CAS; (d) G. Johnson, R. K. Murmann, R. Deavin and W. P. Griffith, Inorg. Synth., 2007, 19, 140 CrossRef.
- J. L. Ferreira da Silva, M. F. Minas da Piedade and M. T. Duarte, Inorg. Chim. Acta, 2003, 356, 222 CrossRef CAS.
- (a) M. Aureliano and D. C. Crans, J. Inorg. Biochem., 2009, 103, 536 CrossRef CAS; (b) M. Aureliano, Dalton Trans., 2009, 9093 RSC; (c) M. Aureliano, G. Fraqueza and C. A. Ohlin, Dalton Trans., 2013, 42, 11770 RSC.
- J. M. Hughes, W. S. Wise, M. E. Gunter, J. P. Morton and J. Rakovan, Can. Mineral., 2008, 46, 1365 CrossRef CAS and reference cited therein.
- (a) W. Bensch, P. Hug, A. Reller and H. R. Oswald, Mater. Res. Bull., 1989, 24, 403 CrossRef CAS; (b) S. Aschwanden, H. W. Schmalle, A. Reller and H. R. Oswald, Mater. Res. Bull., 1993, 28, 575 CrossRef CAS.
- E. E. Hamilton, P. E. Fanwick and J. J. Wilker, J. Am. Chem. Soc., 2002, 124, 78 CrossRef CAS.
- (a) J. Fuchs, S. Mahjour and J. Pickardt, Angew. Chem., Int. Ed. Engl., 1976, 15, 374 CrossRef; (b) G.-Y. Yang, D.-W. Gao, Y. Chen, J.-Q. Xu, Q.-X. Zeng, H.-R. Sun, Z.-W. Pei, Q. Su, Y. Xing, Y.-H. Ling and H.-Q. Jia, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, 616 Search PubMed.
- V. W. Day, W. G. Klemperer and O. M. Yaghi, J. Am. Chem. Soc., 1989, 111, 4518 CrossRef CAS.
- V. W. Day, W. G. Klemperer and D. J. Maltbie, J. Am. Chem. Soc., 1987, 109, 2991 CrossRef CAS.
- (a) V. W. Day, W. G. Klemperer and O. M. Yaghi, J. Am. Chem. Soc., 1989, 111, 5959 CrossRef CAS; (b) W. G. Klemperer, T. A. Marquart and O. M. Yaghi, Mater. Chem. Phys., 1991, 29, 97 CrossRef CAS; (c) N. Kawanami, T. Ozeki and A. Yagasaki, J. Am. Chem. Soc., 2000, 122, 1239 CrossRef CAS; (d) T. Kurata, Y. Hayashi and K. Isobe, Chem. Lett., 2010, 39, 708 CrossRef CAS.
- D. Hou, K. D. Hagen and C. L. Hill, J. Am. Chem. Soc., 1992, 114, 5864 CrossRef CAS.
- D. Hou, K. S. Hagen and C. L. Hill, J. Chem. Soc., Chem. Commun., 1993, 426 RSC.
- J. Marrot, K. Barthelet, C. Simonnet and D. Riou, C. R. Chim., 2005, 8, 971 CrossRef CAS.
- (a) Y. Hayashi, N. Miyakoshi, T. Shinguchi and A. Uehara, Chem. Lett., 2001, 170 CrossRef CAS; (b) K. Okaya, T. Kobayashi, Y. Koyama, Y. Hayashi and K. Isobe, Eur. J. Inorg. Chem., 2009, 5156 CrossRef CAS and references cited therein; (c) J. Forster, B. Rösner, M. M. Khusniyarov and C. Streb, Chem. Commun., 2011, 47, 3114 RSC.
- (a) A. Müller, E. Krickemeyer, M. Penk, H.-J. Walberg and H. Bögge, Angew. Chem., Int. Ed., 1987, 26, 1045 CrossRef; (b) A. Müller, M. Penk, R. Rohlfing, E. Krickemeyer and J. Döring, Angew. Chem., Int. Ed. Engl., 1990, 29, 926 CrossRef; (c) G. B. Karet, Z. Sun, W. E. Streib, J. C. Bollinger, D. N. Hendrickson and G. Christou, Chem. Commun., 1999, 2249 RSC.
- Y. Chen, X. Gu, J. Peng, Z. Shi, H. Yu, E. Wang and N. Hu, Inorg. Chem. Commun., 2004, 7, 705 CrossRef CAS.
- M. I. Khan, S. Ayesh, R. J. Doedens, M. Yu and C. J. O'Connor, Chem. Commun., 2005, 4658 RSC.
- Y. Hayashi, K. Fukuyama, T. Takatera and A. Uehara, Chem. Lett., 2000, 770 CrossRef.
- A. Müller, R. Sessoli, E. Krickemeyer, H. Bögge, J. Meyer, D. Gatteschi, L. Pardi, J. Westphal, K. Hovemeier, R. Rohlfing, J. Döring, F. Hellweg, C. Beugholt and M. Schmidtmann, Inorg. Chem., 1997, 36, 5239 CrossRef.
- A. Müller, E. Krickemeyer, M. Penk, R. Rohlfing, A. Armatage and H. Bögge, Angew. Chem., Int. Ed. Engl., 1991, 30, 1674 CrossRef.
- L. Suber, M. Bonamico and V. Fares, Inorg. Chem., 1997, 36, 2030 CrossRef CAS.
- A. Müller, R. Rohlfing, J. Döring and M. Penk, Angew. Chem., Int. Ed. Engl., 1991, 30, 588 CrossRef.
- See for example (a) K. Hegetschweiler, B. Morgenstern, J. Zubieta, P. J. Hagrman, N. Lima, R. Sessoli and F. Totti, Angew. Chem., Int. Ed., 2004, 43, 3436 CrossRef CAS; (b) I. S. Tidmarsh, R. H. Laye, P. R. Brearley, M. Shanmugam, E. C. Sañudo, L. Sorace, A. Caneschi and E. J. L. McInnes, Chem. Commun., 2006, 2560 RSC; (c) R. H. Laye, Q. Wei, P. V. Mason, M. Shanmugam, S. J. Teat, E. K. Brechin, D. Collison and E. J. L. McInnes, J. Am. Chem. Soc., 2006, 128, 9020 CrossRef CAS; (d) I. S. Tidmarsh, E. Scales, P. R. Brearley, J. Wolowska, L. Sorace, A. Caneschi, R. H. Laye and E. J. L. McInnes, Inorg. Chem., 2007, 46, 9743 CrossRef CAS; (e) I. S. Tidmarsh, R. H. Laye, P. R. Brearley, M. Shanmugam, E. C. Sañudo, L. Sorace, A. Caneschi and E. J. L. McInnes, Chem. – Eur. J., 2007, 13, 6329 CrossRef CAS.
- C.-L. Wang, Z.-B. Zhang, J. Fu, Y. Xu and D.-R. Zhu, Chem. – Eur. J., 2012, 18, 11909 CrossRef CAS.
- G. K. Johnson and E. O. Schlemper, J. Am. Chem. Soc., 1978, 100, 3645 CrossRef CAS.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef.
- See, e.g. B. Dong, C. J. Gómez-García, J. Penga, S. Benmansour and J. Ma, Polyhedron, 2007, 26, 1310 CrossRef CAS.
- (a) B. J. Suh, D. Procissi, P. Kögerler, E. Micotti, A. Lascialfari and F. Borsa, J. Magn. Magn. Mater., 2004, 272–276, e759 CrossRef CAS; (b) D. Rehder, Coord. Chem. Rev., 2008, 252, 2209 CrossRef CAS.
- (a) D. K. Walanda, R. C. Burns, G. A. Lawrance and E. I. von Nagy-Felsobuki, Inorg. Chim. Acta, 2000, 305, 118 CrossRef CAS; (b) Z. L. Chen, G. Owens and R. Naidu, Anal. Chim. Acta, 2007, 585, 32 CrossRef CAS.
- L. A. Truflandier, F. Boucher, C. Payen, R. Hajjar, Y. Millot, C. Bonhomme and N. Steunou, J. Am. Chem. Soc., 2010, 132, 4653 CrossRef CAS.
- S. Ard, C. J. Dibble, S. T. Akin and M. A. Duncan, J. Phys. Chem. C, 2011, 115, 6438 CAS and references cited therein.
- (a) X. Zhang and H. Schwarz, Chem. – Eur. J., 2010, 16, 1163 CrossRef CAS . See, also: ; (b) D. Schröder, M. Engeser, M. Brönstrup, C. Daniel, J. Spandl and H. Hartl, Int. J. Mass Spectrom., 2003, 228, 743 CrossRef; (c) S. Feyel, D. Schröder and H. Schwarz, J. Phys. Chem. A, 2006, 110, 2647 CrossRef CAS; (d) S. Feyel, D. Schröder, X. Rozanska, J. Sauer and H. Schwarz, Angew. Chem., Int. Ed., 2006, 45, 4677 CrossRef CAS; (e) S. Feyel, D. Schröder and H. Schwarz, Eur. J. Inorg. Chem., 2008, 4961 CrossRef CAS.
- I. Lindqvist, Ark. Kemi, 1953, 5, 247 CAS.
- (a) Y. Hayashi, Y. Ozawa and K. Isobe, Chem. Lett., 1989, 425 CrossRef CAS; (b) H. K. Chae, W. G. Klemperer and V. W. Day, Inorg. Chem., 1989, 28, 1423 CrossRef CAS; (c) Q. Chen and J. Zubieta, Inorg. Chem., 1990, 29, 1458 CrossRef; (d) Y. Hayashi, Y. Ozawa and K. Isobe, Inorg. Chem., 1991, 30, 1025 CrossRef CAS; (e) M. I. Khan, Q. Chen, J. Zubieta and D. P. Goshorn, Inorg. Chem., 1992, 31, 1556 CrossRef CAS; (f) Q. Chen, D. P. Goshorn, C. P. Scholes, X. Tan and J. Zubieta, J. Am. Chem. Soc., 1992, 114, 4667 CrossRef CAS; (g) M. I. Khan, Q. Chen, H. Höpe, S. Parkin, C. J. O'Connor and J. Zubieta, Inorg. Chem., 1993, 32, 2929 CrossRef CAS; (h) D. Hou, G.-S. Kim, K. S. Hagen and K. L. Hill, Inorg. Chim. Acta, 1993, 211, 127 CrossRef CAS; (i) M. I. Khan and J. Zubieta, Prog. Inorg. Chem., 1995, 43, 1 CrossRef CAS; (j) A. Müller, J. Meyer, H. Bögge, A. Stammler and A. Botar, Z. Anorg. Allg. Chem., 1995, 621, 1818 CrossRef; (k) M. Piepenbrink, M. U. Triller, N. H. J. Gorman and B. Krebs, Angew. Chem., Int. Ed., 2002, 41, 2523 CrossRef CAS; (l) J. Spandl, C. Daniel, I. Brüdgam and H. Hartl, Angew. Chem., Int. Ed., 2003, 42, 1163 CrossRef CAS; (m) C. Daniel and H. Hartl, J. Am. Chem. Soc., 2005, 127, 13978 CrossRef CAS; (n) M. A. Augustyniak-Jabokow, C. Daniel, H. Hartl, J. Spandl and Y. V. Yablokov, Inorg. Chem., 2008, 47, 322 CrossRef; (o) C. Daniel and H. Hartl, J. Am. Chem. Soc., 2009, 131, 5101 CrossRef CAS.
- J. F. Keggin, Proc. R. Soc. London, Ser. A, 1934, 144, 75 CrossRef CAS.
- (a) R. Kato, A. Kobayashi and Y. Sasaki, J. Am. Chem. Soc., 1980, 102, 6572 CrossRef; (b) R. Kato, A. Kobayashi and Y. Sasaki, Inorg. Chem., 1982, 21, 240 CrossRef CAS; (c) S. Nakamura, T. Yamawaki, K. Kusaka, T. Otsuka and T. Ozeki, J. Cluster Sci., 2006, 17, 245 CrossRef CAS.
- J. M. Maestre, J. M. Poblet, C. Bo, N. Casañ-Pastor and P. Gomez-Romero, Inorg. Chem., 1998, 37, 3444 CrossRef CAS.
- A. Müller, J. Döring, H. Bögge and E. Krickemeyer, Chimia, 1988, 42, 300 Search PubMed.
- T. Yamase, M. Suzuki and K. Ohtaka, J. Chem. Soc., Dalton Trans., 1997, 2463 RSC.
- M. T. Pope, Nature, 1992, 355, 27 CrossRef.
- T. H. Noh, E. Heo, K. H. Park and O.-S. Jung, J. Am. Chem. Soc., 2011, 133, 1236 CrossRef CAS.
- (a) K. Hiratani, M. Goto, Y. Nagawa, K. Kasuga and K. Fujiwara, Chem. Lett., 2000, 1364 CrossRef CAS; (b) J. Garric, J.-M. Léger and I. Huc, Chem. – Eur. J., 2007, 13, 8454 CrossRef CAS; (c) H. J. Shin, J. H. Jung, K. Motobayashi, S. Yanagisawa, Y. Morikawa, Y. Kim and M. Kawai, Nat. Mater., 2010, 9, 442 CrossRef CAS.
- (a) J. Cioslowski and E. D. Fleischmann, J. Chem. Phys., 1991, 94, 3730 CrossRef CAS; (b) J. Cioslowski, J. Am. Chem. Soc., 1991, 113, 4139 CrossRef CAS; (c) Y. Chai, T. Guo, C. Jin, R. E. Haufler, L. P. F. Chibante, J. Fure, L. Wang, J. M. Alford and R. E. Smalley, J. Phys. Chem., 1991, 95, 7564 CrossRef CAS; (d) M. Saunders, H. A. Jimenez-Vazquez, R. J. Cross, S. Mroczkowski, M. L. Gross, D. E. Giblin and R. J. Poreda, J. Am. Chem. Soc., 1994, 116, 2193 CrossRef CAS; (e) J. Cioslowski and Q. Lin, J. Am. Chem. Soc., 1995, 117, 2553 CrossRef CAS; (f) Y. H. Hu and E. Ruckenstein, J. Am. Chem. Soc., 2005, 127, 11277 CrossRef CAS; (g) M. Yamada, T. Nakahodo, T. Wakahara, T. Tsuchiya, Y. Maeda, T. Akasaka, M. Kako, K. Yoza, E. Horn, N. Mizorogi, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 2005, 127, 14570 CrossRef CAS; (h) Z. Ge, J. C. Duchamp, T. Cai, H. W. Gibson and H. C. Dorn, J. Am. Chem. Soc., 2005, 127, 16292 CrossRef CAS; (i) Y. Takano, S. Obuchi, N. Mizorogi, R. García, M. Á. Herranz, M. Rudolf, S. Wolfrum, D. M. Guldi, N. Martín, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2012, 134, 16103 CrossRef CAS; (j) S. Maki, E. Nishibori, I. Terauchi, M. Ishihara, S. Aoyagi, M. Sakata, M. Takata, H. Umemoto, T. Inoue and H. Shinohara, J. Am. Chem. Soc., 2013, 135, 918 CrossRef CAS.
- X. Fang, P. Kögerler, L. Isaacs, S. Uchida and N. Mizuno, J. Am. Chem. Soc., 2009, 131, 432 CrossRef CAS.
- K. Kurotobi and Y. Murata, Science, 2011, 333, 613 CrossRef CAS.
- (a) P. N. W. Baxter, in Comprehensive Supramolcular Chemistry, ed. J. L. Atwood, J. E. D. Davies, D. D. MacNicol and F. Vögtle, Pergamon/Elsevier, New York, 1996, vol. 9, p. 200 Search PubMed; (b) L. Cronin, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. B. Meyer, Elsevier, Amsterdam, 2004, vol. 7, pp. 1–56 and references cited therein Search PubMed.
- H. Wan, C. Wang, Y. Zhang, H. Miao, S. Zhou and Y. Xu, Inorg. Chem., 2014, 53, 10498 CrossRef CAS.
- (a) A. Müller, E. Diemann, E. Krickemeyer and S. Che, Naturwissenschaften, 1993, 80, 77 CrossRef; (b) M.-M. Rohmer, M. Bénard, J. Maestre and J.-M. Poblet, Coord. Chem. Rev., 1998, 178–180, 1019 CrossRef CAS.
- A. Müller, P. Kögerler and C. Kuhlmann, Chem. Commun., 1999, 1347 RSC.
- (a) K. Kastner, J. T. Margraf, T. Clark and C. Streb, Chem. – Eur. J., 2014, 20, 12269 CrossRef CAS; (b) K. Kastner, J. Forster, H. Ida, G. N. Newton, H. Oshio and C. Streb, Chem. – Eur. J., 2015, 21, 7686 CrossRef CAS.
- T. Kurata, A. Uehara, Y. Hayashi and K. Isobe, Inorg. Chem., 2005, 44, 2524 CrossRef CAS.
- K. Yu. Monakhov, O. Linnenberg, P. Kozłowski, J. van Leusen, C. Besson, T. Secker, A. Ellern, X. López, J. M. Poblet and P. Kögerler, Chem. – Eur. J., 2015, 21, 2387 CrossRef.
- J. Tucher, K. Peuntinger, J. T. Margraf, T. Clark, D. M. Guldi and C. Streb, Chem. – Eur. J., 2015, 21, 8716 CrossRef CAS.
- A. Müller, H. Reuter and S. Dillinger, Angew. Chem., Int. Ed. Engl., 1995, 34, 2328 CrossRef.
- J. M. Clemente-Juan, E. Coronado and A. Gaita-Ariño, Chem. Soc. Rev., 2012, 41, 7464 RSC and references cited therein.
- (a) M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, Berlin, 1983 Search PubMed; (b) See special issues on POMs: Chem. Rev., 1998, 98, 1; Chem. Soc. Rev., 2012, 22, 7325; (c) P. Day and E. Coronado, in Magnetism: Molecules to Materials, ed. J. S. Miller and M. Drillon, Wiley-VCH, Weinheim, 2005vol. 5, p. 105 Search PubMed; (d) D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford University Press, Oxford, 2006 Search PubMed.
- See e.g. S. L. Castro, Z. Sun, C. M. Grant, J. C. Bollinger, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 1998, 120, 2365 CrossRef CAS.
- E. M. Zueva, S. A. Borshch, M. M. Petrova, H. Chermette and A. M. Kuznetsov, Eur. J. Inorg. Chem., 2007, 4317 CrossRef CAS.
- C. J. Calzado, J. M. Clemente-Juan, E. Coronado, A. Gaita-Arino and N. Suaud, Inorg. Chem., 2008, 47, 5889 CrossRef CAS.
- W. L. Queen, J. P. West, J. Hudson and S.-J. Hwu, Inorg. Chem., 2011, 50, 11064 CrossRef CAS.
- (a) S.-J. Hwu, Chem. Mater., 1998, 10, 2846 CrossRef CAS; (b) W. L. Queen, S.-J. Hwu and L. Wang, Angew. Chem., Int. Ed., 2007, 46, 5344 CrossRef CAS; (c) J. P. West, W. L. Queen, S.-J. Hwu and K. E. Michaux, Angew. Chem., Int. Ed., 2011, 50, 3780 CrossRef CAS.
- F. Gao and R. Hua, Catal. Commun., 2006, 7, 391 CrossRef CAS.
- For the synthesis of K7[NiV13O38]·16H2O see: C. M. Flynn and M. T. Pope, J. Am. Chem. Soc., 1970, 92, 85 CrossRef CAS.
- M. I. Khan, S. Tabussum, C. L. Marshall and M. K. Neylon, Catal. Lett., 2006, 112, 1 CrossRef CAS.
- (a) M. I. Khan, S. Deb and C. L. Marshall, Catal. Lett., 2009, 128, 256 CrossRef; (b) M. I. Khan, S. Deb, K. Aydemir, A. A. Alwarthan, S. Chattopadhyay, J. T. Miller and C. L. Marshall, Catal. Lett., 2010, 135, 282 CrossRef CAS; (c) M. I. Khan, K. Aydemir, M. R. H. Siddiqui, A. A. Alwarthan and C. L. Marshall, Catal. Lett., 2011, 141, 538 CrossRef CAS . See also ; (d) S. Albonetti, F. Cavani and F. Trifiro, Catal. Rev.: Sci. Eng., 1996, 38, 413 CrossRef CAS; (e) M. D. Argyle, K. Chen, A. T. Bell and E. Iglesia, J. Catal., 2002, 208, 139 CrossRef CAS; (f) M. A. Bañares and S. J. Khatib, Catal. Today, 2004, 96, 251 CrossRef; (g) O. R. Evans, A. T. Bell and T. D. Tilley, J. Catal., 2004, 226, 292 CrossRef CAS.
- Q. Wu, X. Hao, X. Feng, Y. Wang, Y. Li, E. Wang, X. Zhu and X. Pan, Inorg. Chem. Commun., 2012, 22, 137 CrossRef CAS.
- S. Chakrabarty and R. Banerjee, Catal. Sci. Technol., 2012, 2, 2224 CAS.
- N. Kato and Y. Hayashi, Dalton Trans., 2013, 42, 11804 RSC.
- M.-P. Santoni, G. La Ganga, V. M. Nardo, M. Natali, F. Puntoriero, F. Scandola and S. Campagna, J. Am. Chem. Soc., 2014, 136, 8189 CrossRef CAS.
- Y. Gao, Y. Chi and C. Hu, Polyhedron, 2014, 83, 242 CrossRef CAS.
- R. C. Wheland, J. Am. Chem. Soc., 1976, 98, 3926 CrossRef CAS.
- (a) D. Gatteschi, L. Pardi, A. L. Barra and A. Müller, Mol. Eng., 1993, 3, 157 CrossRef CAS; (b) G. Chaboussant, S. T. Ochsenbein, A. Sieber, H.-U. Güdel, H. Mutka, A. Müller and B. Barbara, Europhys. Lett., 2004, 66, 423 CrossRef CAS; (c) P. Kögerler, B. Tsukerblat and A. Müller, Dalton Trans., 2010, 39, 21 RSC; (d) B. Tsukerblat and A. Tarantul, in Molecular Cluster Magnets, ed. R. Winpenny, World Scientific Publishers, Singapore, 2011, ch. 3 Search PubMed.
- K. Ariga, J. P. Hill and Q. Ji, in Supramolecular Soft Matter: Applications in Materials and Organic Electronics, ed. T. Nakanishi, John Wiley & Sons, Hoboken, NJ, 2011 Search PubMed.
- (a) B. Nohra, H. El Moll, L. M. Rodriguez Albelo, P. Mialane, J. Marrot, C. Mellot-Draznieks, M. O'Keeffe, R. Ngo Biboum, J. Lemaire, B. Keita, L. Nadjo and A. Dolbecq, J. Am. Chem. Soc., 2011, 133, 13363 CrossRef CAS; (b) P. Van Der Voort, K. Leus, Y.-Y. Liu, M. Vandichel, V. Van Speybroeck, M. Waroquier and S. Biswas, New J. Chem., 2014, 38, 1853 RSC.
- X. Wang, L. Liu, G. Zhang and A. J. Jacobson, Chem. Commun., 2001, 2472 RSC.
- Y. Gao, Y. Xu, Y. Cao and C. Hu, Dalton Trans., 2012, 41, 567 RSC.
- A. Tripathi, T. Hughbanks and A. Clearfield, J. Am. Chem. Soc., 2003, 125, 10528 CrossRef CAS.
- Y. Gao, Y. Xu, S. Li, Z. Han, Y. Cao, F. Cui and C. Hu, J. Coord. Chem., 2010, 63, 3373 CrossRef CAS.
- D. Pitzschke, J. Wang, R.-D. Hoffmann, R. Pöttgen and W. Bensch, Angew. Chem., Int. Ed., 2006, 45, 1305 CrossRef CAS.
- T. Whitfield, X. Wang and A. J. Jacobson, Inorg. Chem., 2003, 42, 3728 CrossRef CAS.
- J. Wang, C. Näther, P. Kögerler and W. Bensch, Inorg. Chim. Acta, 2010, 363, 4399 CrossRef CAS.
- J. Wang, C. Näther, P. Kögerler and W. Bensch, Eur. J. Inorg. Chem., 2012, 1237 CrossRef CAS.
- Y.-M. Chen, E.-B. Wang, B.-Z. Lin and S.-T. Wang, Dalton Trans., 2003, 519 RSC.
- L.-H. Bi, U. Kortz, M. H. Dickman, S. Nellutla, N. S. Dalal, B. Keita, L. Nadjo, M. Prinz and M. Neumann, J. Cluster Sci., 2006, 17, 143 CrossRef CAS.
- H. T. Evans Jr. and J. A. Konnert, Am. Mineral., 1978, 63, 863 Search PubMed.
- A. Müller, J. Döring, M. I. Khan and V. Wittneben, Angew. Chem., Int. Ed., 1991, 30, 210 CrossRef.
- (a) N. Suaud, Y. Masaro, E. Coronado, J. M. Clemente-Juan and N. Guihéry, Eur. J. Inorg. Chem., 2009, 5109 CrossRef CAS; (b) S. Cardona-Serra, J. M. Clemente-Juan, A. Gaita-Ariño, N. Suaud, O. Svoboda and E. Coronado, Chem. Commun., 2013, 49, 9621 RSC.
- (a) B. Keita, K. Essaadi, L. Nadjo, R. Contant and Y. Justum, J. Electroanal. Chem., 1996, 404, 271 CrossRef; (b) B. Keita, I.-M. Mbomekalle, L. Nadjo, P. de Oliveira, A. Ranjbari and R. Contant, C. R. Chim., 2005, 8, 1057 CrossRef CAS.
- L.-S. You, Q.-Y. Zhu, X. Zhang, Y.-Y. Pu, G.-Q. Bian and J. Dai, CrystEngComm, 2013, 15, 2411 RSC.
- J. Zhou, J. Zhang, W.-H. Fang and G.-Y. Yang, Chem. – Eur. J., 2010, 16, 13253 CrossRef CAS.
- (a) B. Botar, P. Kögerler, A. Müller, R. Garcia-Serres and C. L. Hill, Chem. Commun., 2005, 5621 RSC; (b) B. Botar, A. Ellern, M. T. Sougrati and P. Kögerler, Eur. J. Inorg. Chem., 2009, 5071 CrossRef CAS.
- J. Zhou, J.-W. Zhao, Q. Wei, J. Zhang and G.-Y. Yang, J. Am. Chem. Soc., 2014, 136, 5065 CrossRef CAS.
- Y. Gao, Y. Xu, K. Huang, Z. Han and C. Hu, Dalton Trans., 2012, 41, 6122 RSC.
- J. Wang, C. Näther, M. Speldrich, P. Kögerler and W. Bensch, CrystEngComm, 2013, 15, 10238 RSC.
- (a) A. Müller, M. Penk and J. Döring, Inorg. Chem., 1991, 30, 4935 CrossRef; (b) A. L. Barra, D. Gatteschi, B. S. Tsukerblatt, J. Döring, A. Müller and L. C. Brunel, Inorg. Chem., 1992, 31, 5132 CrossRef CAS.
- M. I. Khan, Q. Chen and J. Zubieta, Inorg. Chim. Acta, 1993, 212, 199 CrossRef CAS.
- G.-Q. Huang, S.-W. Zhang, Y.-G. Wei and M.-C. Shao, Polyhedron, 1993, 12, 1483 CrossRef CAS.
- (a) W.-M. Bu, L. Ye, G.-Y. Yang, M.-C. Shao, Y.-G. Fan and J.-Q. Xu, Chem. Commun., 2000, 1279 RSC; (b) Y. Zhao, Q. Liu, Y. Li, X. Chen and Z. Mai, J. Mater. Chem., 2001, 11, 1553 RSC.
- A. Müller, J. Döring and H. Bögge, J. Chem. Soc., Chem. Commun., 1991, 273 RSC.
- D. Gatteschi, B. Tsukerblatt, A. L. Barra, L. C. Brunel, A. Müller and J. Döring, Inorg. Chem., 1993, 32, 2114 CrossRef CAS.
- R. Basler, G. Chaboussant, A. Sieber, H. Andres, M. Murrie, P. Kögerler, H. Bögge, D. C. Crans, E. Krickemeyer, S. Janssen, H. Mutka, A. Müller and H.-U. Güdel, Inorg. Chem., 2002, 41, 5675 CrossRef CAS.
- A. Wutkowski, N. Evers and W. Bensch, Z. Anorg. Allg. Chem., 2011, 637, 2205 CrossRef CAS.
- S.-T. Zheng, J. Zhang and G.-Y. Yang, Eur. J. Inorg. Chem., 2004, 2004 CrossRef CAS.
- S.-T. Zheng, M.-H. Wang and G.-Y. Yang, Inorg. Chem., 2007, 46, 9503 CrossRef CAS.
- Y. Qi, Y. Li, E. Wang, Z. Zhang and S. Chang, Dalton Trans., 2008, 2335 RSC.
- Y. Qi, Y. Li, C. Qin, E. Wang, H. Jin, D. Xiao, X. Wang and S. Chang, Inorg. Chem., 2007, 46, 3217 CrossRef CAS.
- S.-T. Zheng, J. Zhang and G.-Y. Yang, Inorg. Chem., 2005, 44, 2426 CrossRef CAS.
- D. Zhao, S.-T. Zheng and G.-Y. Yang, J. Solid State Chem., 2008, 181, 3071 CrossRef CAS.
- X.-B. Cui, J.-Q. Xu, H. Meng, S.-T. Zheng and G.-Y. Yang, Inorg. Chem., 2004, 43, 8005 CrossRef CAS.
- A. Müller and J. Döring, Z. Anorg. Allg. Chem., 1991, 595, 251 CrossRef.
- G. Huan, M. A. Greaney and A. J. Jacobson, J. Chem. Soc., Chem. Commun., 1991, 260 RSC.
- S.-T. Zheng, J. Zhang and G.-Y. Yang, Z. Anorg. Allg. Chem., 2005, 631, 170 CrossRef CAS.
- S.-T. Zheng, J. Zhang and G.-Y. Yang, J. Mol. Struct., 2004, 705, 127 CrossRef CAS.
- G. Zhou, Y. Xu, C. Guo, Y. Liu and X. Zheng, J. Cluster Sci., 2007, 18, 388 CrossRef CAS.
- X.-B. Cui, J.-Q. Xu, Y. Li, Y.-H. Sun, L. Ye and G.-Y. Yang, J. Mol. Struct., 2003, 657, 397 CrossRef CAS.
- S.-Y. Shi, Y. Chen, J.-N. Xu, Y.-C. Zou, X.-B. Cui, Y. Wang, T.-G. Wang, J.-Q. Xu and Z.-M. Gao, CrystEngComm, 2010, 12, 1949 RSC.
- E. Dumas, C. Livage, S. Halut and G. Hervé, Chem. Commun., 1996, 2437 RSC.
- S.-T. Zheng, J. Zhang and G.-Y. Yang, J. Mol. Struct., 2005, 752, 25 CrossRef CAS.
- S.-T. Zheng, J.-Q. Xu and G.-Y. Yang, J. Cluster Sci., 2005, 16, 23 CrossRef CAS.
- (a) Y.-F. Qi, Y. Li, E. Wang, D. Xiao and J. Hua, J. Coord. Chem., 2007, 60, 1403 CrossRef CAS; (b) G. Zhou, C. Guo, W. Liu, Y. Xu and X. Zheng, J. Coord. Chem., 2008, 61, 202 CrossRef CAS.
- C. Wang, G. Zhou, Z. Zhang, D. Zhu and Y. Xu, J. Coord. Chem., 2011, 64, 1198 CrossRef CAS.
- A. Kondinski, T. Heine and K. Yu. Monakhov, 2015, submitted.
- Y. Qi, Y. Li, E. Wang, H. Jin, Z. Zhang, X. Wang and S. Chang, Inorg. Chim. Acta, 2007, 360, 1841 CrossRef CAS.
- S.-T. Zheng, J. Zhang, J.-Q. Xu and G.-Y. Yang, J. Solid State Chem., 2005, 178, 3740 CrossRef CAS.
- X.-B. Cui, J.-Q. Xu, Y. Li, Y.-H. Sun and G.-Y. Yang, Eur. J. Inorg. Chem., 2004, 1051 CrossRef CAS.
- Y. Qi, Y. Li, E. Wang, H. Jin, Z. Zhang, X. Wang and S. Chang, J. Solid State Chem., 2007, 180, 382 CrossRef CAS.
- C.-M. Wang, Q.-X. Zeng, J. Zhang and G.-Y. Yang, J. Cluster Sci., 2005, 16, 65 CrossRef CAS.
- B. Dong, J. Peng, C. J. Gómez-García, S. Benmansour, H. Jia and N. Hu, Inorg. Chem., 2007, 46, 5933 CrossRef CAS.
- X.-B. Cui, Y.-Q. Sun and G.-Y. Yang, Inorg. Chem. Commun., 2003, 6, 259 CrossRef CAS.
- T. Arumuganathan and S. K. Das, Inorg. Chem., 2009, 48, 496 CrossRef CAS.
- A. Müller and J. Döring, Angew. Chem., Int. Ed. Engl., 1988, 27, 1721 CrossRef.
- G.-Y. Yang, L.-S. Chen, J.-Q. Xu, Y.-F. Li, H.-R. Sun, Z.-W. Pei, Q. Su, Y.-H. Lin and Y. Xing, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, 1556 Search PubMed.
- X.-B. Cui, J.-Q. Xu, L. Ding, H. Ding, L. Ye and G.-Y. Yang, J. Mol. Struct., 2003, 660, 131 CrossRef CAS.
- S.-T. Zheng, Y.-M. Chen, J. Zhang and G.-Y. Yang, Z. Anorg. Allg. Chem., 2006, 632, 155 CrossRef CAS.
- S.-T. Zheng, J. Zhang and G.-Y. Yang, Chem. Lett., 2003, 32, 810 CrossRef CAS.
- S.-T. Zheng, Y.-M. Chen, J. Zhang, J.-Q. Xu and G.-Y. Yang, Eur. J. Inorg. Chem., 2006, 397 CrossRef CAS.
- W.-M. Bu, G.-Y. Yang, L. Ye, J.-Q. Xu and Y.-G. Fan, Chem. Lett., 2000, 462 CrossRef CAS.
- X.-B. Cui, J.-Q. Xu, Y.-H. Sun, Y. Li, L. Ye and G.-Y. Yang, Inorg. Chem. Commun., 2004, 7, 58 CrossRef CAS.
- S.-T. Zheng, J. Zhang, B. Li and G.-Y. Yang, Dalton Trans., 2008, 5584 RSC.
- G. Huan, J. W. Johnson, A. J. Jacobson and J. S. Merola, Chem. Mater., 1990, 2, 719 CrossRef CAS.
- M. I. Khan, Y. Chang, Q. Chen, H. Hope, S. Parkin, D. P. Goshorn and J. Zubieta, Angew. Chem., Int. Ed. Engl., 1992, 31, 1197 CrossRef.
- M. I. Khan and J. Zubieta, Angew. Chem., Int. Ed. Engl., 1994, 33, 760 CrossRef.
- L. Zhang and W. Schmitt, J. Am. Chem. Soc., 2011, 133, 11240 CrossRef CAS.
- O. Delgado, A. Dress, A. Müller and M. T. Pope, Mol. Eng., 1993, 3, 9 CrossRef CAS.
- J. M. Breen, L. Zhang, R. Clement and W. Schmitt, Inorg. Chem., 2012, 51, 19 CrossRef CAS.
- J. M. Breen and W. Schmitt, Angew. Chem., Int. Ed., 2008, 47, 6904 CrossRef CAS.
- (a) J. S. Anderson, Nature, 1937, 140, 850 CrossRef CAS; (b) H. T. Evans Jr., J. Am. Chem. Soc., 1948, 70, 1291 CrossRef; (c) A. Bridgeman and G. Cavigliasso, Inorg. Chem., 2002, 41, 1761 CrossRef CAS; (d) A. Bridgeman and G. Cavigliasso, J. Phys. Chem. A, 2003, 107, 6613 CrossRef CAS.
- (a) I. Lindqvist, Acta Crystallogr., 1950, 3, 159 CrossRef CAS; (b) B. Courcot and A. J. Bridgeman, J. Phys. Chem. A, 2009, 113, 10540 CrossRef CAS.
- K. Yu. Monakhov, C. Gourlaouen, R. Pattacini and P. Braunstein, Inorg. Chem., 2012, 51, 1562 CrossRef CAS.
- I. Chiorescu, W. Wernsdorfer, A. Müller, H. Bögge and B. Barbara, J. Magn. Magn. Mater., 2000, 221, 103 CrossRef CAS.
- G. Chaboussant, R. Basler, A. Sieber, S. T. Ochsenbein, A. Desmedt, R. E. Lechner, M. T. F. Telling, P. Kögerler, A. Müller and H.-U. Güdel, Europhys. Lett., 2002, 59, 291 CrossRef CAS.
- D. Procissi, A. Lascialfari, E. Micotti, M. Bertassi, P. Carretta, Y. Furukawa and P. Kögerler, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 184417 CrossRef.
- I. Chiorescu, W. Wernsdorfer, A. Müller, S. Miyashita and B. Barbara, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 020402 CrossRef.
- G. Chaboussant, S. T. Ochsenbein, A. Sieber, H.-U. Güdel, H. Mutka, A. Müller and B. Barbara, Europhys. Lett., 2004, 66, 423 CrossRef CAS.
- Y. Furukawa, Y. Nishisaka, K. Kumagai, P. Kögerler and F. Borsa, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 220402 CrossRef.
- A. Tarantul, B. Tsukerblat and A. Müller, Inorg. Chem., 2007, 46, 161 CrossRef CAS.
- S. Bertaina, S. Gambarelli, T. Mitra, B. Tsukerblat, A. Müller and B. Barbara, Nature, 2008, 453, 203 CrossRef CAS.
- A. Tarantul and B. Tsukerblat, Inorg. Chim. Acta, 2010, 363, 4361 CrossRef CAS.
- D. Gatteschi, L. Pardi, A. L. Barra and A. Müller, Mol. Eng., 1993, 3, 157 CrossRef CAS.
- (a) I. Chiorescu, W. Wernsdorfer, A. Müller, H. Bögge and B. Barbara, Phys. Rev. Lett., 2000, 84, 3454 CrossRef CAS; (b) K. Kajiyoshi, T. Kambe, M. Mino, H. Nojiri, P. Kögerler and M. Luban, J. Magn. Magn. Mater., 2007, 310, 1203 CrossRef CAS.
- (a) D. W. Boukhvalov, V. V. Dobrovitski, M. I. Katsnelson, A. I. Lichtenstein, B. N. Harmon and P. Kögerler, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 054417 CrossRef; (b) M. Machida and S. Miyashita, Physica E, 2005, 29, 538 CrossRef.
- D. Gatteschi, L. Pardi, A. L. Barra, A. Müller and J. Döring, Nature, 1991, 354, 463 CrossRef CAS.
- D. W. Boukhvalov, V. V. Dobrovitski, M. I. Katsnelson, A. I. Lichtenstein, B. N. Harmon and P. Kögerler, J. Appl. Phys., 2003, 93, 7080 CrossRef CAS.
- X.-X. Hu, J.-Q. Xu, X.-B. Cui, J.-F. Song and T.-G. Wang, Inorg. Chem. Commun., 2004, 7, 264 CrossRef CAS.
- R. Kiebach, C. Näther and W. Bensch, Solid State Sci., 2006, 8, 964 CrossRef CAS.
- R. Kiebach, C. Näther, P. Kögerler and W. Bensch, Dalton Trans., 2007, 3221 RSC.
- A. Wutkowski, C. Näther, P. Kögerler and W. Bensch, Inorg. Chem., 2008, 47, 1916 CrossRef CAS.
- E. Antonova, C. Näther, P. Kögerler and W. Bensch, Angew. Chem., Int. Ed., 2011, 50, 764 CrossRef CAS.
- Y. Gao, Z. Han, Y. Xu and C. Hu, J. Cluster Sci., 2010, 21, 163 CrossRef CAS.
- E. Antonova, C. Näther, P. Kögerler and W. Bensch, Dalton Trans., 2012, 41, 6957 RSC.
- L. Zhang, X. Zhao, J. Xu and T. Wang, J. Chem. Soc., Dalton Trans., 2002, 3275 RSC.
- E. Antonova, C. Näther and W. Bensch, CrystEngComm, 2012, 14, 6853 RSC.
- E. Antonova, A. Wutkowski, C. Näther and W. Bensch, Solid State Sci., 2011, 13, 2154 CrossRef CAS.
- E. Antonova, C. Näther, P. Kögerler and W. Bensch, Inorg. Chem., 2012, 51, 2311 CrossRef CAS.
- H. Lühmann, C. Näther, P. Kögerler and W. Bensch, Inorg. Chim. Acta, 2014, 421, 549 CrossRef.
- E. Antonova, C. Näther and W. Bensch, Dalton Trans., 2012, 41, 1338 RSC.
- A. Wutkowski, C. Näther, P. Kögerler and W. Bensch, Inorg. Chem., 2013, 52, 3280 CrossRef CAS.
- (a) S. Albonetti, F. Cavani, F. Trifiro, M. Gazzano, F. C. Aissi, A. Aboukais and M. J. Guelton, J. Catal., 1994, 146, 491 CrossRef CAS; (b) S. Albonetti, F. Cavani, M. Koutyrev and F. Trifiro, Catal. Lett., 1995, 30, 253 CrossRef; (c) F. Cavani, M. Koutyrev and F. Trifiro, Catal. Today, 1995, 24, 365 CrossRef CAS; (d) F. Cavani, M. Koutyrev and F. Trifiro, Catal. Today, 1996, 28, 319 CrossRef CAS; (e) F. Cavani, A. Tanguy, F. Trifiro and M. Koutrev, J. Catal., 1998, 174, 231 CrossRef CAS.
- J. Spengler, F. Anderle, E. Bosch, R. K. Grasselli, B. Pillep, P. Behrens, O. B. Lapina, A. A. Shubin, H.-J. Eberle and H. Knözinger, J. Phys. Chem. B, 2001, 105, 10772 CrossRef CAS.
- M. Schindler, F. C. Hawthorne and W. H. Baur, Chem. Mater., 2000, 12, 1248 CrossRef CAS.
- R. B. King, THEOCHEM, 1995, 336, 165 CrossRef CAS.
- (a) A. B. Bourlinos, K. Raman, R. Herrera, Q. Zhang, L. A. Archer and E. P. Giannelis, J. Am. Chem. Soc., 2004, 126, 15358 CrossRef CAS; (b) P. G. Rickert, M. R. Antonio, M. A. Firestone, K.-A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, J. Phys. Chem. B, 2007, 111, 4685 CrossRef CAS; (c) P. G. Rickert, M. R. Antonio, M. A. Firestone, K.-A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, Dalton Trans., 2007, 529 RSC; (d) S. Herrmann, M. Kostrzewa, A. Wierschem and C. Streb, Angew. Chem., Int. Ed., 2014, 13596 CrossRef CAS; (e) S. Herrmann, A. Seliverstov and C. Streb, J. Mol. Eng. Mater., 2014, 2, 1440001 CrossRef.
- S. S. Mal, O. Tröppner, I. Ivanović-Burmazović and P. Burger, Eur. J. Inorg. Chem., 2013, 1960 CrossRef CAS.
- A. Proust, R. Thouvenot and P. Gouzerh, Chem. Commun., 2008, 1837 RSC.
- Y. V. Geletii, B. Botar, P. Kögerler, D. A. Hillesheim, D. G. Musaev and C. L. Hill, Angew. Chem., Int. Ed., 2008, 47, 3896 CrossRef CAS.
- (a) J. Tucher, L. C. Nye, I. Ivanovic-Burmazovic, A. Notarnicola and C. Streb, Chem. – Eur. J., 2012, 18, 10949 CrossRef CAS; (b) J. Tucher and C. Streb, Beilstein J. Nanotechnol., 2014, 5, 711 CrossRef CAS.
- C. Aronica, G. Chastanet, E. Zueva, S. A. Borshch, J. M. Clemente-Juan and D. Luneau, J. Am. Chem. Soc., 2008, 130, 2365 CrossRef CAS.
- (a) M.-M. Rohmer and M. Bénard, J. Am. Chem. Soc., 1994, 116, 6959 CrossRef CAS; (b) M.-M. Rohmer, J. Devemy, R. Wiest and M. Bénard, J. Am. Chem. Soc., 1996, 118, 13007 CrossRef CAS; (c) C. Menke, E. Diemann and A. Müller, J. Mol. Struct., 1997, 436–437, 35 CrossRef CAS . See also; (d) J. M. Poblet, X. López and C. Bo, Chem. Soc. Rev., 2003, 32, 297 RSC; (e) X. López, J. J. Carbó, C. Bo and J. M. Poblet, Chem. Soc. Rev., 2012, 41, 7537 RSC.
- (a) M. R. S. A. Janjua, Z.-M. Su, W. Guan, A. Irfan, S. Muhammad and M. Iqbal, Can. J. Chem., 2010, 88, 434 CrossRef CAS; (b) V. V. Maslyuk, I. Mertig, O. V. Farberovich, A. Tarantul and B. Tsukerblat, Eur. J. Inorg. Chem., 2013, 1897 CrossRef CAS.
- D. Rehder, Dalton Trans., 2013, 42, 11749 RSC.
- (a) D. Rehder, Bioinorganic Vanadium Chemistry, John Wiley & Sons, Hoboke, NJ, 2008, pp. 13–51 Search PubMed. See, also: ; (b) B. D. Wladkowski, L. A. Svensson, L. Sjolin, J. E. Ladner and G. L. Gilliland, J. Am. Chem. Soc., 1998, 120, 5488 CrossRef CAS.
- (a) E. G. DeMaster and R. A. Mitchell, Biochemistry, 1973, 12, 3616 CrossRef CAS; (b) E. F. Pai, W. Sachsenheimer, R. H. Schirmer and G. E. Schulz, J. Mol. Biol., 1977, 114, 37 CrossRef CAS; (c) G. Choate and T. E. Mansour, J. Biol. Chem., 1979, 254, 11457 CAS; (d) G. Soman, Y. C. Chang and D. J. Graves, Biochemistry, 1983, 22, 4994 CrossRef CAS; (e) D. W. Boyd, K. Kustin and M. Niwa, Biochim. Biophys. Acta, 1985, 827, 472 CrossRef CAS; (f) D. C. Crans, K. Sudhakar and T. J. Zamborelli, Biochemistry, 1992, 31, 6812 CrossRef CAS.
- N. Steens, A. M. Ramadan, G. Absillis and T. N. Parac-Vogt, Dalton Trans., 2010, 39, 585 RSC.
- S. S. Soares, C. Gutiérrez-Merino and M. Aureliano, J. Inorg. Biochem., 2007, 101, 789 CrossRef CAS PubMed.
- H. Stephan, M. Kubeil, F. Emmerling and C. E. Müller, Eur. J. Inorg. Chem., 2013, 1585 CrossRef CAS PubMed.
- (a) N. D. Chasteen, Struct. Bonding, 1983, 53, 105 CrossRef CAS; (b) D. C. Crans, in Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity, ed. A. Müller and M. T. Pope, Kluwer Academic Publishers, Dordrecht, 1993, pp. 399–406 Search PubMed.
- Vanadium Compounds, Chemistry, Biochemistry, and Therapeutic Applications, ed. A. S. Tracey and D. C. Crans, American Chemical Society, Washington, DC, 1998, p. 711 Search PubMed.
- (a) D. C. Crans, R. L. Bunch and L. A. Theisen, J. Am. Chem. Soc., 1989, 111, 7597 CrossRef CAS; (b) D. C. Crans, J. J. Smee, E. Gaidamauskas and L. Yang, Chem. Rev., 2004, 104, 849 CrossRef CAS PubMed; (c) M. Aureliano, World J. Biol. Chem., 2011, 2, 215 CrossRef PubMed and references cited therein; (d) S. Ramos, J. J. G. Moura and M. Aureliano, Metallomics, 2012, 4, 16 RSC.
- (a) J. Stankiewicz, A. S. Tracey and D. C. Crans, in Vanadium and Its Role in Life: Metal Ions in Biological Systems, ed. H. Sigel and A. Sigel, Marcel Dekker, New York, 1995, vol. 31, pp. 287–324 Search PubMed; (b) A. Bishayee, A. Waghray, M. A. Patel and M. Chatterjee, Cancer Lett., 2010, 294, 1 CrossRef CAS PubMed.
- E. Antonova, B. Seidlhofer, J. Wang, M. Hinz and W. Bensch, Chem. – Eur. J., 2012, 18, 15316 CrossRef CAS PubMed.
- (a) J. Fielden, K. Quasdorf, L. Cronin and P. Kögerler, Dalton Trans., 2012, 41, 9876 RSC; (b) B. Botar, A. Ellern and P. Kögerler, Dalton Trans., 2012, 41, 8951 RSC; (c) B. Botar, A. Ellern, R. Hermann and P. Kögerler, Angew. Chem., Int. Ed., 2009, 48, 9080 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |