Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Redefining biorefinery: the search for unconventional building blocks for materials
Davide
Esposito
* and
Markus
Antonietti
Max-Planck-Institute of Colloids and Interfaces, 14424 Potsdam, Germany. E-mail: davide.esposito@mpikg.mpg.de
First published on 24th April 2015
Abstract
This review discusses different strategies for the upgrading of biomass into sustainable monomers and building blocks as scaffolds for the preparation of green polymers and materials.
Key learning points(1) Biorefinery provides access to non-conventional building blocks and scaffolds for the preparation of new sustainable materials and polymers.(2) Chemical strategies for the deconstruction of lignocellulosic biomass in its carbohydrate and lignin fractions can be generally classified into degradative or non-degradative processes. (3) Hydrothermal and solvothermal methods can be combined with catalytic processes to generate specific monomers or oligomers as sustainable scaffolds for material science. (4) The materials and polymers synthesized on the basis of biomass derived molecules present different and extended properties compared with the ones conventionally obtained from fossil resources. (5) Biorefinery can generally increase the sustainability of current (nano)technologies. |
Introduction
The core of the chemical industry is the conversion of raw materials into value-added products, including fuels, platform chemicals, polymers, materials and pharmaceuticals. In the last century, fossil resources have constituted the primary feedstock for a multitude of chemical processes and transformations. However, sustainability is becoming a global imperative, due to the considerable depletion of fossil resources, and major scientific and political players have been promoting the paradigm of “biorefinery” as a solution for sustainable development. In a biorefinery, renewable feedstocks (biomass or food waste) are refined/upgraded to yield fuels and commodity chemicals by means of chemical and biological conversion technologies.In the past few years, many biorefinery studies, propelled by the availability of specific funding schemes, have focused on the production of energy in the form of fuels. As a consequence, the scientific literature has witnessed the proliferation of different studies describing methodologies to convert biopolymers from lignocellulosic biomass into hydrocarbon fuels, pyrolytic bio-oils and fuel additives.1 Nevertheless, after the first wave of enthusiasm, the role of biofuels in a future energy supply scenario has been subjected to intensive criticisms, especially due to the so called “food vs. fuel” competition, as well as an overall less favorable energy balance. From an economic perspective, it is important to consider that classical fuels and energy carriers are produced with the minimal leverage as methane (“biogas”), ethanol (“biofuel”) or “biodiesel”. However, chemists are usually aware that there is more to chemical molecules than their energy content, as demonstrated by the continuous search for new “materials and medicines”. This is why attention has been slowly re-directed to the development of routes for the conversion of lignocellulose and waste streams into an interesting platform and specialty chemicals. These highly valuable and marketable products are usually required in smaller volumes if compared to fuels. At such scales, the existing side-products from well-established sectors (e.g. agroindustry, food sector, paper manufacturing and recycling) can efficiently serve as the renewable feedstock for the production of specialty chemicals and materials. Therefore, the upgrade of biomass waste can be performed in a substantially environmental friendly way, avoiding pressure on agriculture to switch to the production of specific crops with the resulting problems associated with land use changes, conflicts with the food markets and loss of biodiversity, which are the strongest problems associated with the production of biofuels.2 More in general, producing chemicals from waste streams of established sectors has the potential to additionally raise the value of the latter without altering or refocusing the current production.
In the last decades, a considerable number of reviews have appeared in an attempt to catalogue the many different strategies developed to convert the three major bio polymers contained in biomass into conventional base chemicals. Here, the economic competition is quite high, and an effective market penetration is still uncertain, since classical fossil-based production chains are well established and quite economical. In addition, competition is expected to increase due the controversial shale gas revolution, which seems capable of providing some of the traditional energy carriers and platform chemicals at a very competitive price, despite the highly adverse environmental effects.3
Besides those conventional tracks, biorefinery approaches, however, have brought focus on a series of building blocks that are unique and potential game changing alternatives to the present building blocks used ubiquitously by the chemical industry for the preparation of materials and pharmaceuticals. Such molecules do not compete immediately with petrochemicals and might be used for new and innovative products. Lactic acid, levulinic acid, 5-hydroxymethylfurfural, γ-valerolactone as well as lignin derivatives have received the attention of the scientific community since they represent a new pool of building blocks or chemical space, which in many cases can be employed with minimal derivatization for the production of new materials, polymers and pharmaceuticals. The great potential of the obtained products is the ability to offer alternative and improved properties with respect to the corresponding oil-derived specialty products, which in many cases even go beyond the intrinsic advantages of sustainability and biocompatibility.
This review intends to provide a short overview on the latest developments and trends in the preparation of new materials and polymers based on those “less traditional” chemicals obtained from biorefinery. We will initially describe selected examples of chemical biorefinery approaches which allow for the recovery of valuable building blocks from the direct treatment of raw biomass. A representative selection of novel materials synthesized on the basis of such building blocks will be presented, with the goal of demonstrating how green and sustainable processes can positively impact the field of material and polymer chemistry. Emphasis is placed on some aspects of the synthetic methodologies of these materials that further contribute to the sustainability of the final products.
General considerations on biorefinery
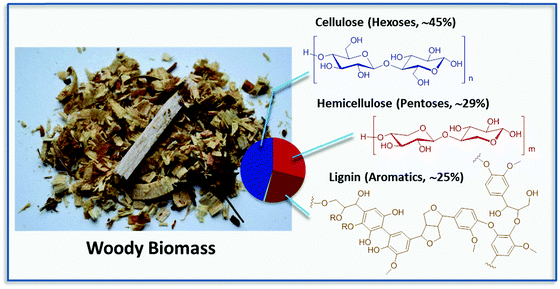
Biorefinery has been often compared to traditional refinery, which converts fossil crude oil into higher value products by optimized processes capable of valorizing each fraction of the complete crude. Some of the most relevant differences are that oil is found in nature in the form of a liquid and is characterized by low oxygen content, while biomass is often solid and composed to a high extent of oxygenated molecules. Raw materials including organic crop waste, wood and straw are highly heterogeneous and are mostly composed of polysaccharides (usually accounting for 60–80% of the weight) and the aromatic rich lignin (15–30 wt%), which are tightly bound in the form of a nanocomposite (Fig. 1).Due to the complication of processing, the initial approach to the biorefinery of woody biomass was focusing on a first step turning solids into processable liquids, i.e., onto the production of so-called biocrude oils (e.g., pyrolysis oils, hydrothermally derived oils), which are being already commercialized by a number of companies around the world (see for instance Licella Ltd). However, these bio-oils are not really competitive if the final goal is the valorization into higher products, e.g., building blocks and functional molecules. The here advised selective generation of fine and platform chemicals has proven much more demanding and troublesome from a technical point of view. Due to the high heterogeneity of biomass, any process aimed at biomass deconstruction into its monomers acts on all of the components at any one time generating often broad mixtures of compounds. The cost of separating the components of these mixtures has been one of the major drawbacks of such approaches. Hence, the development of more selective methodologies for the deconstruction of lignocellulose and the separation of its constituents is of crucial importance.
A convenient solution to circumvent this challenge could be to separate the carbohydrate portion of the biomass from the lignin, thereby producing two streams of products ready for further processing. Solvothermal methods, i.e., the treatment of biomass with solvents at elevated temperatures, have found broad application, due to their simplicity, adaptability to wet biomass, and environmental compatibility. Many research groups have optimized these approaches for the sole isolation of the cellulose or lignin fraction, inspired by the knowledge on pulping processes developed by the paper industry. In the past few years, however, new methodologies that allow for the simultaneous deconstruction of polysaccharides and the partial depolymerization of lignin have been designed. From now on we will refer to them as degradative (DD) and non-degradative (NDD) deconstruction processes. NDD processes are usually based on mild dissolution treatments with either organic solvents (“organosolve” processes) or water, and with the auxiliary of mild additives (catalyst, reactants or derivatization reagents). In this kind of processes, based on the difference of solubility in the chosen solvent system under the applied conditions, cellulose, hemicellulose and lignin can be separated in the form of partially depolymerized substances, which can be further deconstructed at a later stage. In DD processes, catalysts or reactants are applied that can promote hydrolysis and cleavage reactions to a higher extent. By appropriate selection of the catalytic conditions, DD processes can be optimized to generate a variety of different products ranging from mixtures of partially and fully depolymerized carbohydrates and lignin to well defined building blocks and platform chemicals (Fig. 2). In addition, it is important to remember that besides chemical approaches, the repertoire of biorefinery can comprise a series of very efficient biotransformation processes, which are alternatively capable of converting NDD products into building blocks which complement and expand the pool of compounds obtainable by DD processes.
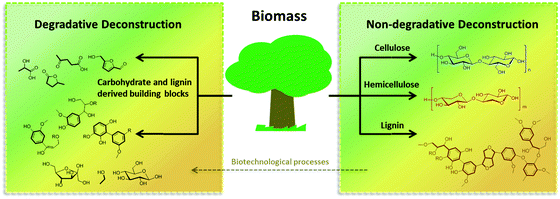 | ||
| Fig. 2 A representation of degradative (DD) and non-degradative (NDD) deconstruction processes, for the valorization of biomass. | ||
The following describes some particularly interesting building blocks that can be obtained via DD and NDD processes followed by representative examples of their application in the field of material science. The goal will be to guide the reader through examples that indicate the interplay of green chemistry and material sciences, while a comprehensive description of the field is clearly out of the possibilities of such a tutorial.
Lactic acid
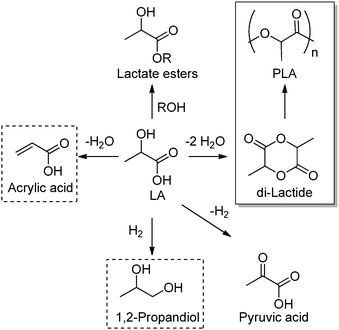
Lactic acid (LA) or 2-hydroxypropanoic acid is an important C-3 platform chemical targeted by biorefinery. Besides its direct application as a food additive or a component of pharmaceutical and cosmetic formulations, LA can be regarded as the precursor of a series of chemicals of enormous industrial importance. Simple catalytic transformations which have been applied to LA include oxidation to pyruvic acid, reduction to 1,2-propandiol, or esterification to generate a series of lactate esters (Scheme 1). In the field of polymer science, the dehydration of LA into acrylic acid has been explored, but the synthesis of di-lactide, used for the preparation of the biodegradable polylactic acid (PLA), remains the most important applications of LA. Many reviews have recently been published summarizing the most recent advances in lactic acid production and transformation.4,5Traditionally, fermentation is the preferred option for the production of LA in industry. This approach relies on the use of glucose and bacteria and, in comparison to classical chemical processes, the main drawbacks are the low space-time productivity, the cost of separation, and massive aqueous side products. Ideally, the direct chemical conversion of raw lignocellulosic substances could substantially simplify and increase the sustainability of LA production. For this reason, several groups have recently reported different approaches for the chemical synthesis of LA. In many cases, models have been used in order to acquire a mechanistic understanding of the processes. Trioses in particular have been identified as an intermediate in the synthesis of LA and were exploited as starting materials for the preparation of the latter under a variety of different conditions. Although very valuable for the theoretical understanding, such approaches are penalized by the use of expensive trioses, and for a detailed description of such methods we redirect the reader to the literature.4 Here, the direct conversion of crude lignocellulosic biomass or cellulose into LA is outlined, which presents clear economic advantages. Preliminary studies have recently showcased the value and efficacy of hydrothermal conversion of carbohydrate precursors. Additional investigations to unravel the fundamentals of carbohydrate deconstruction and identify specific conditions that avoid the generation of mixtures of products are the subject of current research. In the contest of LA production, the alkaline hydrothermal treatment of cellulose has shown promising results. While the decomposition of carbohydrates into LA under basic conditions has been known for long time, the ability of hydrothermal conditions to accelerate the formation of LA has been only rediscovered recently. The addition of additives during the hydrothermal deconstruction of cellulose plays a crucial role in enhancing LA yield and selectivity.5 Capitalizing on different studies which analyzed the role of NaOH, ZnSO4 and NiSO4 during the hydrothermal hydrolysis of glucose, a batch method was recently developed which combines the use of Ni, Zn and activated carbon in NaOH solutions (2.5 M) and is capable of converting cellulose into LA in only five minutes with a yield of 42% at 300 °C.6 Alternatively, Ca(OH)2 was successfully used for the production of LA from corn cobs, affording the latter in 45% yield after treatment at 300 °C.7 Recently, our group developed a competitive approach for the base catalyzed hydrothermal treatment of biomass based on the use of Ba(OH)2.8 Such treatment results in the controlled depolymerization of the cellulosic portion of biomass with the preferential formation of barium lactate. These DD conditions allow for the convenient separation of the lignin contained in the biomass in the form of low molecular weight oligomers (∼1 kDa). Thanks to its hydrophobic nature, lignin fragments can be easily recovered by simple filtration at the end of the reaction after precipitation. The decomposition of sugars under basic conditions is assumed to proceed via a retro-aldol splitting of the carbon backbone (Scheme 2) resulting in the formation of glyceraldehyde and dihydroxyacetone in dynamic equilibrium. Dehydration to methylglyoxal followed by a 1,2-hydride shift finally affords the lactate fragment, which can be neutralized to the corresponding LA by acids.
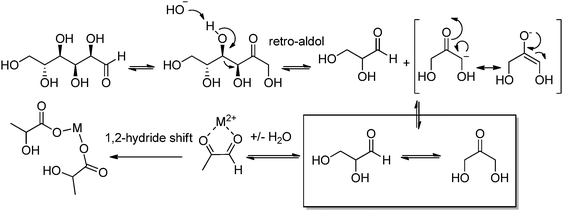 | ||
| Scheme 2 Mechanism of LA formation via basic hydrolysis of sugar in water. Reproduced with permission from ref. 8. Copyright 2013, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The use of acidic conditions has also been recently proposed for the synthesis of LA in superheated water. Unlike alkaline treatments, such approaches bypass the need for an acidic workup and the loss of reagents in neutralization. The group of Dong recently reported an intriguing protocol for the preparation of lactic acid from cellulose based on the use of lanthanide triflates, with europium triflate showing the best activity and a yield of ca. 63%.9 In this case, the reaction was performed at 240 °C on a 300 mg scale for 30 minutes under an overpressure of nitrogen (20 bar). Interestingly, recycling experiments successfully showed the possible reuse of the catalyst over at least five reaction cycles. A second interesting report, which appeared almost simultaneously, described the efficient preparation of LA (68% yield) using Pb(II) ions at even milder temperatures (190 °C, 30 bar N2, 4 h) starting from cellulose.10 The authors also reported the possibility of converting raw biomass under the same conditions, leading to the isolation of LA in 40% yield.
Lactic acid in material sciences
A major segment of the annual LA production is dedicated to the synthesis of di-lactide, the cyclic dimer of self-condensation of LA which is in turn the starting material for the synthesis of high molecular weight PLA (Scheme 1). Although PLA can also be directly obtained via polycondensation of LA, this reaction only leads to lower molecular weight polymers. Such oligolactides are usually the precursor of dilactide via catalytic backbiting. The preparation of PLA has been extensively covered in previous reviews, as well as its application in the packaging, pharmaceutical and biomedical sectors.5 PLA is characterized by relatively limited thermal and mechanical properties, and different research groups have been recently working on the preparation of blends and copolymers of LA as well as composites with improved properties. In order to preserve the green footprint of the final material, copolymerization of LA with monomers or compounds which can be as well obtained from biomass via DD and NDD processes are currently under investigation. In this regard, the preparation of PLA composites with cellulose nanowhiskers represents a very interesting example. Ahmed and Thielemans recently reported the preparation of PLA fibers whose tensile modulus increases of about 45% via addition of cellulose nanowhiskers at their surface using polyvinyl acetate as the binding agent.11 This particular treatment additionally confers to the PLA fiber surface an increased roughness and hydrophilic character. Moreover, the nanocomposite showed improved biocompatibility and functioned well as support for cell proliferation, anticipating the application of this class of composites for biomedical uses. Besides cellulose fibers and whiskers, lignin is another potential biomass component for such composites. The groups of Sattely and Waymouth reported an interesting study on the preparation of PLA–lignin copolymers obtained by grafting PLA onto lignin during organocatalyzed lactide ring opening polymerization. The obtained copolymers display expanded thermal properties compared to commercial PLA. In addition, due to the UV active aromatic motifs introduced by the lignin fragments, the final material was characterized by improved optical properties.12From levulinic acid towards materials
Levulinic acid (LEV) is a γ-keto acid obtainable via DD of cellulosic materials. LEV is one of the most studied products in the contest of biorefinery, both in academia as well as in industry (it was obtained over the past century by treatment of raw biomass with very strong inorganic acids), and it was one of the first platform chemical targeted for commercial applications (see the Biofine process). Nowadays, this commodity is practically obtained via acid catalyzed hydrothermal hydrolysis of cellulose, which proceeds with the simultaneous formation of stoichiometric amounts of formic acid, together with insoluble carbonaceous condensation products called humins. LEV represents a promising candidate for the generation of a series of other platform chemicals, and possible conversion strategies have been extensively discussed.1 Among others, LEV has been proposed as a starting material for the synthesis of useful esters, pyrrolidones, diols and cyclic lactones, some of which are depicted in Scheme 3. Among all possible products obtainable from LEV, γ-valerolactone (GVL) has received the most attention for its role as renewable solvent and sustainable fuel additive.13 This compound is characterized by a very low toxicity and it has a similar polarity to dimethylformamide, which makes it a remarkably good medium for the synthesis of macromolecules as well as for the deconstruction, fractionation and hydrolysis of biomass polymers. Different approaches have been recently proposed to increase the efficiency of GVL preparation. In many cases, new catalyst preparations are sought that are characterized by improved stability towards strong acidic environments. In this way, the direct processing of crude LEV feeds obtained by hydrolysis of biomass is facilitated, although very often the use of rare transition metals has been required. Alternatively, emphasis is given to the development of green protocols for the selective extraction of LEV from acidic aqueous solutions. This approach allows performing the successive hydrogenation in the absence of acid and enables the use of commercially available catalysts based on non-rare transition metals.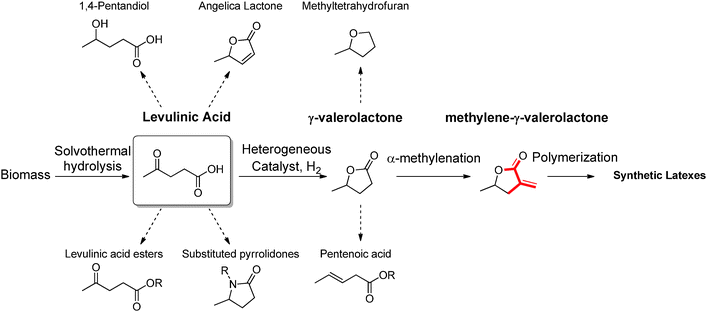 | ||
| Scheme 3 Potential routes for the conversion of levulinic acid into building blocks, solvents and monomers for synthetic polymers. | ||
Besides its potential as green solvent, GVL represents an interesting scaffold for the preparation of polymers, since it can be further functionalized affording polymerizable monomers. Among other strategies, α-methylenation is exploited to prepare methylene-γ-valerolactone (Scheme 3), which is amenable to radical polymerization and could mimic the reactivity of more classical petrochemical (meth)acrylates.
In the past, similar vinyl butyrolactones were already considered as suitable candidates to potentially expand the family of methyl methacrylate polymers. The presence of a locked ring structure in such monomers increases the reactivity in free radical polymerizations. In addition, vinyl butyrolactones derived polymers show a higher refractive index and higher glass transition temperatures, which can be considered advantageous. α-Methylene-γ-butyrolactone (MBL), for example, is a natural vinyl monomer which can be extracted from tulips. Different methodologies have been applied for the preparation of polymers based on this compound, which performs well in radical and anionic polymerization. Poly-α-methylene-butyrolactone is characterized by a glass transition temperature of 195 °C and proved to be stable up to 320 °C, while offering improved solvent resistance if compared to poly(methyl methacrylate) due to the presence of the lactone ring that increases chain rigidity.14 Nevertheless, direct extraction of MBL from plants can only be performed with low yields. Currently, materials based on this compound can only be obtained by synthesizing the monomer on the basis of oil derived platform chemicals. In contrast, MGVL could become available on the basis of a sustainable biorefinery process (Scheme 3). Routes to access MGVL are still subject of research, as the α-methylenation of γ-valerolactone has not yet been systematically optimized. Recently, Manzer reported the preparation of MGVL from levulinic acid using a two-step approach, consisting of a Ru/C catalyzed hydrogenation, followed by the gas phase (340 °C) α-methylenation of the resulting γ-valerolactone in the presence of silica supported barium acetate.15 The main drawback of this approach is the recurring deactivation of the catalyst during the reaction. For this reason, the solution phase methylenation of GVL could represent a valuable alternative. Molinari et al. recently introduced a scheme for the acid catalyzed DD of biomass aimed at the formation of LEV, followed by RANEY® Ni catalyzed reduction of LEV to γ-valerolactone, which is made possible by an extractive protocol performed in methyl tetrahydrofuran.16 Such organic feeds could be in principle used as the starting material for the solution methylenation of GVL using paraformaldehyde under basic conditions. The products isolated via this approach are expected to be amenable to radical solution and heterophase polymerization opening the way to strategies for the preparation of high molecular weight latexes under a variety of different conditions (Fig. 3). Interestingly, MGVL polymers have already been reported in the literature previously. Stille et al. for instance, described the synthesis of MGVL from non-sustainable precursors (propylene oxide and 1-bromovinyl trimethylsilane) followed by examples of radical and ionic polymerization of MGVL, which afforded amorphous polymers characterized by very high Tg (211–220 °C).17 More recently, the group of Jones reported the emulsion and microemulsion polymerization of MGVL as a convenient way to obtain high molecular weight polymers.18 As anticipated, this class of materials has the potential to expand the range of applicability of acrylates, due to the improved thermal stability.
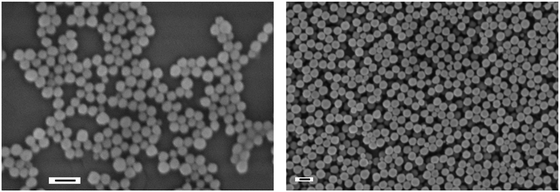 | ||
| Fig. 3 Scanning electron micrographs of PMGVL latexes prepared with KPS (PDL, left) and AIBN (ADL, right); the bars indicate 200 nm. | ||
5-Hydroxymethylfurfural and related products and materials
Besides LEV, acid catalyzed DD processes offer convenient opportunities for the preparation of 5-hydroxymethylfurfural (HMF) as a direct dehydration product of carbohydrates. Obtained also as a byproduct during the hydrolysis of biomass into LEV, the selective production of HMF has only been achieved more recently, because of its tendency to generate condensation of byproducts and humins as insoluble precipitates, especially for reactions at high starting concentrations. Similar mechanisms have been evoked in the genesis of hydrothermal carbon from lignocellulose and have been reviewed elsewhere.19 During DD processes of biomass, HMF is generated as the product of dehydration of the intermediate fructose, formed in situ via glucose isomerization. In practice, as the quantitative isomerization of glucose to fructose is rather complicated, HMF is often synthesized in high yields using fructose as the starting material. Fructose can be obtained as the product of enzymatic upgrade of starch and inuline. A number of different solutions have been proposed to convert fructose into HMF, with most methods relying on the use of organic solvents. In general, such transformations can be performed with high yields (90% to quantitative) under rather mild conditions, usually not exceeding temperatures of 120 °C, and in short times (∼2 h).Among different solvents, dimethyl sulfoxide (DMSO) has shown the highest performance for the solvolysis of sugars into HMF. It has been speculated that DMSO can act as an effective stabilizer for the furanose form of fructose, which is the active species undergoing dehydration to HMF.20 For this reason, the reaction can be performed also at higher concentrations, albeit excessive loadings (50 wt% solutions) result in a considerable decrease in the yield (∼50%). The alternative use of DMSO in the mixture with other solvents (e.g., acetone), or the use of different solvents systems based on dimethylformamide has shown good results. In combination with such a solvent system, a variety of acidic catalysts have been evaluated. In general, sulfonic acid based catalysts have shown very promising results, in both their homogeneous and heterogeneous form. Also the use of zeolites and heteropolyacids has been explored and details in this direction can be found in ref. 20. An alternative approach for the generation of HMF is based on the use of ionic liquids (ILs). In this contest, imidazolium based ionic liquids proved particularly effective in promoting the formation of HMF, with ethyl methyl imidazolium chloride being one of the best solvents.21 These ionic solvents showed their best performance in combination with chromium salts. Such systems could even enable the direct conversion of glucose and cellulose into HMF with reasonable preparative yields (ca. 45%). Nevertheless, due to the high cost of production of ILs and the high environmental impact associated with the use of chromium salts, these methods are still undergoing continuing development, and the quest for cheaper ILs and less toxic catalysts is still ongoing. Finally, it is worth mentioning that although the conversion of fructose into HMF appears to be quite robust and reliable, the direct use of raw cellulosic biomass is preferred, due to the higher costs of fructose compared with raw cellulosic materials. In this regard, the development of catalysts for the effective isomerization of glucose into fructose will play a pivotal role. Examples from the field of zeolites have appeared. The group of Davis for instance, has recently reported the development of tin-based zeolites capable of converting glucose into fructose under very mild conditions with high efficiency.22 Nevertheless, these processes go beyond the scope of this review and will not be discussed in detail.
HMF conveniently conserves the 6-carbon backbone common to its carbohydrate precursor, but is at the same time characterized by half of the oxygen content. The pendant hydroxyl as well as the aldehyde functionality can be easily modified by means of catalytic transformations. In general, derivatives of HMF have found application either as monomers for polymers, or as fuel additives and fuel precursors if the oxygen content is appropriately further reduced, as shown in Scheme 4. The types of transformations which are commonly applied on HMF usually involve reduction and oxidation reactions. The development of new sustainable catalytic procedures for the upgrade of HMF, which are compatible with industrial implementation, is currently a very active area of research. Particular emphasis is given to the engineering of new catalysts which are based on abundant and inexpensive metals, with the intention of replacing the more classical rare transition metal based catalysts. Examples of approaches leading to the generation of more sustainable catalysts will be briefly given in the next section. Here, we would like to recall some general features of representative and established catalytic schemes for the derivatization of HMF which have recently appeared in the literature (a more general review on this topic has been recently published by Corma et al.1). HMF oxidation can occur on both its alcohol and aldehyde functionalities, with the possibility of forming intermediate mono-oxidation products, resulting for instance in monoacid species. One of the most interesting and well-studied derivatives of HMF is the so called 2,5-furandicarboxylic acid (FDCA, Scheme 4). This di-carboxylic acid was identified as a potential substitute of terephthalic acid for the preparation of PET analogs, due to structural similarity. Several different methods for the oxidation of HMF into FDCA, which rely on the use of noble metals have been proposed. Initially, Pd, Pt and Au based catalysts have provided good results, but a bimetallic catalyst prepared by the addition of a second metal to these well studied systems proved in many cases to show superior activities.23 The reactions are usually performed in water at temperatures between 25 and 100 °C, using dioxygen as the oxidant.24 The latter has the role to regenerate the metal surface by scavenging electrons, thereby forming hydroxide and peroxide ions. The reaction has also shown to be pH dependent, in a basic environment promoting the efficient formation of FDCA. Due to the possible disproportionation of HMF under basic conditions via Cannizzaro reactions, stringent pH control needs to be applied, and generally the use of two equivalents of base represents a reasonable compromise. Besides oxidation products, reduced derivatives of HMF have also been proposed and reported recently. Simple catalytic reduction of the aldehyde on HMF can be performed to generate 2,5-dihydroxymethylfuran (DHMF), a versatile diol which can serve as a useful building block in the preparation of polyesters. DHMF can be prepared using conventional strategies,1,21 which rely on the use of supported transition metals in combination with a reducing agent. Novel catalyst preparation based on alloys has also been recently reported and showed an excellent activity.25 Such alloys were prepared excluding the use of rare transition metals, with a clear advantage in terms of sustainability. Interestingly, the presence of additives can significantly alter the selectivity of hydrogenation resulting in the alternative formation of decarbonylation products, deoxygenation affording 2,5-dimethylfuran (DMF) or even the fully saturated dimethyl tetrahydrofuran (DMTHF). In this regard, the group of Rauchfuss has recently highlighted the versatility of palladium on carbon for such transformations, whereby the selectivity towards DMF and DMTHF can be tuned by selection of the solvent and additives, using formic acid or molecular hydrogen as the hydrogen donor respectively.26 Such reduced derivatives are characterized by excellent solvent properties and have great potential as a diesel substitute and fuel additives. DMF has been also recently exploited for the synthesis of para xylene in combination with shale gas derived ethylene.3
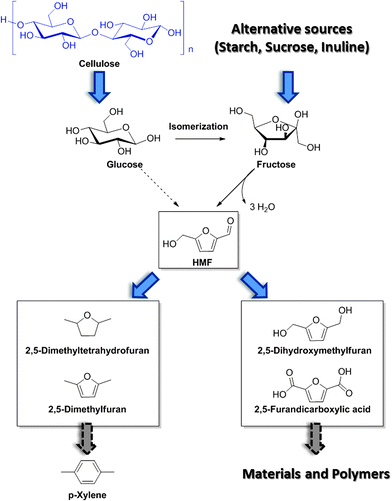 | ||
| Scheme 4 Schematic of HMF synthesis and derivatization into useful building blocks and fuel additives. | ||
All materials and polymers prepared on the basis of the monomers described before are essentially low carbon materials. In this direction, the first idea for sustainable polymers based on furan derivatives came with the preparation of polyethylene furanoate (PEF) as a potential replacement for polyethylene terephthalate (PET), since terephthalic acid has been recently added to the list of potential human endocrine disruptors. This new type of polyester presents considerable advantages in terms of gas permeation (e.g., oxygen permeation) compared to PET. The success of this application has boosted the proliferation of an intense area of research aimed at the synthesis of additional classes of polymeric compounds based on sustainable furans. Examples of different polyesters, followed by polyurethane and polyamides, are currently appearing in the scientific literature.27 A comprehensive description of this exciting and rapidly developing area however goes beyond the scope of this tutorial presentation.
Cellulose derived materials
Many of the primary products from non-degradative treatments of biomass can be conveniently upgraded into different materials by straightforward transformations. Cellulose represents a very well established and consolidated example in this direction. The paper industry has applied NDD processes known as “pulping” since long time. Such processes (e.g., soda pulping, Kraft-process, etc.) which commonly make use of relatively mild conditions are at the basis for the production of cellulose based fibers like viscose and cellophane. A more detailed description of such pulping approaches will be given in the next section. Here, we would like to underline the fact that in the past, despite a considerable initial success, the production of cellulose based materials was hampered by the development of analogous and more economical materials obtained on the basis of oil precursors. Recently, the necessity of eliminating non-renewable resources from the production chains of some commodity materials as well as the establishment of the biorefinery paradigm have newly stimulated research aimed at the preparation of new cellulose based materials characterized by novel properties. In the previous paragraph for instance, we mentioned how cellulose nanowhiskers have been recently exploited in the field of polymer composites.11 As excellent studies have been already done describing these developments, we only mention this branch for completeness.Besides polymer composites, another traditional usage of cellulose is carbonization for the synthesis of biocarbons and activated carbons. These carbons are practical and cheap choices to support metal nanoparticles affording in this way catalysts that can be successfully applied in the field of hydrogenolysis and hydrogenation. Such processes are actually crucial for the success of biorefinery, since many of the primary products of DD as well as NDD treatments of biomass can be upgraded by straightforward catalytic transformations, as discussed above. Unfortunately, very often these transformations rely on very expensive catalysts based on rare metals from the platinum-group. In addition, such supported catalysts are often subject to leaching and passivation when in contact with real biomass hydrolysates. The use of inexpensive and abundant elements, for example iron and nickel, in combination with cheap and accessible supports prepared by cellulose carbonization can result in an optimal combination for the preparation of heterogeneous catalysts. Such catalysts have the potential to mitigate the costs for the production of sustainable chemicals from biomass. Cellulose paper has been recently used as a structure precursor for the preparation of carbon electrodes under preservation of the macro- and microtexture.28 Inspired by such an approach, carbon supported alloys have also been prepared by simple impregnation of cellulose filter paper with nickel and iron nitrates, followed by carbothermal reduction.25 This form of reductive treatment is particularly indicated for the generation of carbon encapsulated nanoparticles (Fig. 4).
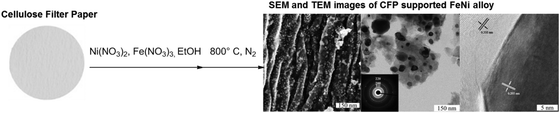 | ||
| Fig. 4 Schematic representation of carbon supported FeNi nanoparticles obtained by carbothermal reduction of cellulose. Figure adapted from ref. 25, © 2014. | ||
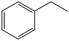
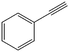
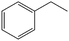
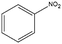
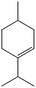
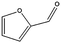
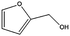
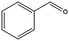
The encapsulation of FeNi nanoparticles into graphene layers provides a highly stable yet very active material, which was successfully tested as a catalyst for the continuous hydrogenation of different molecules, including compounds obtained by the DD treatments of biomass (Table 1).
| Entry | Substrate | Product | Conversion–selectivityc (%–%) | T (°C) |
|---|---|---|---|---|
| Conditions: 0.1 M solution of starting material in EtOH.a 1 mL min−1, 10 bar (H2).b 0.3 mL min−1, 50 bar (H2).c Determined by uncorrected GC-MS analysis. | ||||
| 1a |
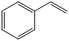
|
|
>99–>99 | 50 |
| 2a |
|
|
>99–>99 | 50 |
| 3a |
|
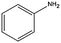
|
>99–>99 | 100 |
| 4a |
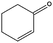
|
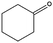
|
>99–>99 | 100 |
| 5a |
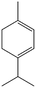
|
|
>99–56 | 125 |
| 6a |
|
|
>99–90 | 150 |
| 7a |
|
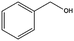
|
>99–84 | 150 |
| 8a |
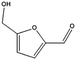
|
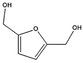
|
>99–88 | 150 |
| 9b |
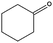
|
|
84–>99 | 150 |
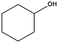
| 10b |
|
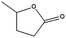
|
99–60 | 150 |
| 11a |
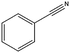
|
|
>99–40 | 150 |
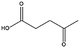
The catalyst showed high activity for the reduction of HMF for instance, but also for molecules like glucose and xylose which in industry are traditionally converted into the corresponding alditols using RANEY® nickel. Interestingly, the carbon supported NiFe alloy showed a prolonged stability on stream (ca. 80 h).
Besides catalytic materials, an array of different functional materials can be prepared by using cellulose as base. A particularly noteworthy example is the preparation of magnetic paper based on nano-cellulose.29 In this case the cellulose fibrils act as a polymeric dispersant for ferromagnetic nanoparticles, which are incorporated into or constructed onto the support via simple sol–gel techniques. The resulting paper can function as a simple actuator in the presence of a magnetic field.
Lignin and lignin derivative procurement
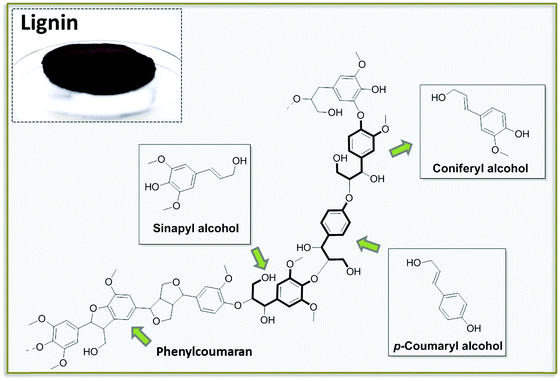
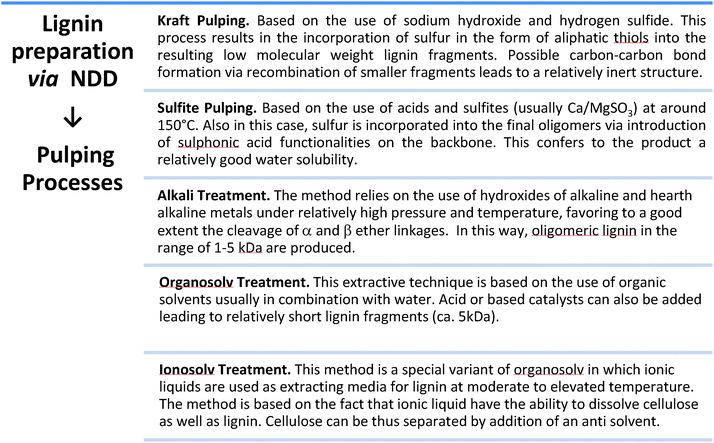
Lignin is one of the main products obtainable via NDD treatments of biomass. This polymeric substance, being a major component of woody biomass, has been historically produced by the paper industry, usually as a side product of pulping processes, converted into energy by incineration. In recent years, lignin has received attention as a potential source for aromatic building blocks. Lignin is biosynthesized in plants via the random interconnection of substituted phenyl propane units. Phenyl propane backbones are common to the three most abundant building blocks of lignin, namely coumaryl, coniferyl and sinapyl alcohols (Fig. 5).30 The condensation of these building blocks into a polymeric structure via a combination of phenol radicals is mediated by peroxidases. The resulting framework is rather heterogeneous, also considering the occurrence of additional building blocks contained in lower amounts (e.g. phenylcoumaran), and it is characterized by the presence of several aryl ether and carbon–carbon bonds with different bond energies (usually classified as 4-O-5, β-O-4, β–β).30 The most abundant functional groups present in lignin are generally aliphatic and phenolic alcohols, together with methoxy groups.Lignin of different compositions can be accessed by proper selection of the biomass. Interestingly, hardwood, softwood and grass lignin incorporate the same pool of monomers, although the relative content of all building blocks is quite different. Softwood lignin is built up to a large extent of coniferyl alcohol, for instance, while hardwood lignin is characterized by the presence of both coniferyl and sinapyl alcohols in comparable amounts. An additional factor which greatly influences the composition of the lignin is the typology of the processing strategy (NDD) that is utilized to separate it from the crude biomass. The characteristics of the most common industrially applied pulping methods for the isolation of lignin have been described before.31 Some of the general features of such treatments are detailed in Fig. 6. The different chemistries underlying these treatments provide lignin fragments with different degrees of polymerization and may promote an alteration in the distribution of functional groups. In addition, the presence of additives employed during the pulping process often results in the introduction of functionalities which are originally not present in the native lignin structure. For instance, in the case of Kraft and Sulfite pulping the isolated lignin incorporates a considerable amount of sulfur in the form of thiol groups. All pulping processes usually afford lignin in the form of low molecular weight polymers or even oligomers.
The further depolymerization of such fragments can be achieved by a second processing step and in this regard different approaches have been presented in the literature, mostly focusing on reductive and oxidative methods.30 Recently, the hydrogenolytic cleavage of aryl ethers linkages promoted by a nickel-based catalyst has received considerable attention. This exciting reaction, which has been initially evaluated on model molecules that reproduce the most common aryl-ether linkages found in lignin, has shown great potential in both its homogeneous and heterogeneous variations. For instance, a new nickel–titanium nitride composite has been developed which catalyzes the cleavage of molecules like diphenyl ether and substituted analogs thereof under relatively mild conditions in the presence of gaseous hydrogen. Such reaction converts diphenyl ether into a stoichiometric mixture of benzene and cyclohexanol, while preliminary experiments performed on a real alkali lignin sample demonstrated the occurrence of depolymerization.32 Similar results have been achieved also using nickel deposited on silica as the catalysts.33 Transfer hydrogenation methodologies that bypass the necessity of working with gaseous hydrogen have also been proposed. Here, lignin depolymerization using formic acid as the hydrogen donor in the presence of Ni deposited on a zeolite support (Al-SBA-15) has shown promising results.34 Alternatively, isopropanol has been exploited as the hydrogen donor in combination with RANEY® Ni for the processing of organosolv lignin, resulting in the generation of non-pyrolitic, non-gumming bio oils. Such crudes contained lignin fragments with a very low degree of polymerization and in which the main backbone has been decomposed to a great extent into mono aromatic units and aliphatic residues.35 A further development of this method was accomplished by Rinaldi et al. who utilized a combination of an organosolv treatment with a simultaneous transfer hydrogenation in one pot, for the direct conversion of biomass. By treating wood with 2-PrOH–H2O water mixtures at 180 °C in the presence of RANEY® Ni they were able to deconstruct the biomass recovering phenol rich non-pyrolitic bio-oils as the result of the lignin depolymerization (Fig. 7).36 In addition, the residual pulp showed high susceptibility to enzymatic hydrolysis for the production of glucose.
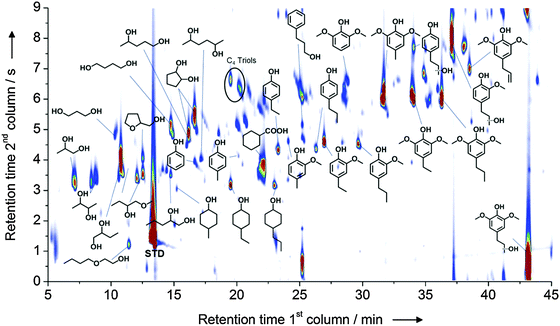 | ||
Fig. 7 Speciation of the products obtained from the catalytic biorefining of poplar wood in the presence of RANEY® Ni at 180 °C for 3 h (2-PrOH/H2O, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). Reproduced with permission from ref. 35. Copyright 2014, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. 3, v/v). Reproduced with permission from ref. 35. Copyright 2014, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In recent years, novel alternative processing methods for the isolation of lignin based on the use of ILs have been proposed. The group of Welton for instance, has recently reviewed the possible use of ionic solvents for the selective extraction of lignin from biomass.37 The approach has shown promising preliminary results and is based on the ability of ionic liquid to dissolve cellulose or in some cases even the whole lignocellulosic biomass. Ionic liquids are also efficient media for different types of nanoparticle catalyzed reactions and in addition have been used for nanocatalyst structuration.38 For this reason, ionothermal extractions of lignin from biomass are promising candidates to be integrated with procedures in which the successive catalytic upgrading or depolymerization of lignin is performed using the same ionic liquid exploited for the extraction, in order to increase compatibility. The main concern associated with this kind of strategy is the relatively high price and non-sustainability of current commercial ionic liquids, although an intense area of research is emerging which focuses on the development of novel sustainable ionic liquids.39
Despite the efficiency of the treatments aimed at the isolation of lignin, one the main drawbacks is still the lack of efficient and robust methods for the separation of the single families of the aromatic compounds contained in the processed lignin fraction. Nevertheless, the phenol rich matrixes isolated via NDD treatments of biomass and subsequent depolymerization represent a unique pool of building blocks for the preparation of renewable colloids and polymers, which can be often utilized for this purpose in the form of semi-crude mixtures. In the following, an interesting representative examples of materials prepared on the basis of lignin fragments will be discussed.
Lignin derived materials and colloids
The direct use of lignin for the generation of polymer particles and nanocomposites as well as the repolymerization of lignin fragments as a strategy for the formation of reconstituted polymers, glues, resins, and binders is indeed thought to be one of the hot areas of green polymer science.Work based on the use of lignin to reinforce polyesters was already mentioned above,12 but also the direct generation of monodisperse polymer filler particles as biobased polymer beads replacing traditional petrochemical polymers is highly interesting and directly applicable.40 The most exciting and direct use of lignin as the active material in a polymer battery and a supercapacitor was however reported by Inganäs et al.41 In their report, they demonstrated that lignin, in a mixture with a cationic conducting polymer used to provide electronic conductivity, can serve as an electron storing material, with an overall performance exceeding the one of traditional, non-sustainable materials by a factor of 3–5. Compared to other petrochemical derived materials, lignin is not only increasing the sustainability of the final device, but also shows the potential to bring clear advantages in performance for a highly relevant and valuable application. This showcases how sustainable molecules open up pathways to materials for sustainable technologies and applications, which do not come with compromised or reduced properties, but in turn have the potential to modify the market as such.
Substituted phenols from the decomposition of wood lignin or other properly-selected biowaste have of course much broader applications. The traditional Japanese art to produce lacquerware which goes under the name of “Urushi” is based on urushiol, the common name for a mixture of monomers sharing a catechol unit functionalized with alkyl tails of different lengths (Fig. 8). These compounds are obtained from the resin of the lacquer tree.
It is clear from our cultural heritage that such lacquer objects are extremely durable and come with a high trade value (although they are allergenic). Substrates which conserve the functionality of urushiol but are characterized by lower allergenic effects could be in principle the product of a specialized biorefining scheme which aims at the preparation of lacquer on the basis of lignin, as discussed above. Cardanol, for instance, which can be isolated from cashew nut shell liquid, presents interesting structural similarity to urushiol. Recently, an intriguing review has appeared which summarizes the most important application of cardanol to the synthesis of polymers and additives.42
Phenols in modern polymer technology are the primary components of phenol-resins, such as resorcinol-formaldehyde (RF)-resins. Such macromolecules are nowadays commonly employed for the preparation of advanced mesoporous sorption materials,43 electrodes and also materials for energy storage.44 In addition, bisphenol derivatives are an essential part of polycarbonates and epoxy-resins, although their use has been the subject of a controversial debate due to toxicity issues. For instance, bisphenol-A (BPA) is known for its severe adverse effect on the human endocrine system.45 In this regard, lignin bioraffinate products with lowered health impact as replacement for BPA and analogous molecules are a safe bet for one of the first blockbusters from modern biorefining schemes. As an example, being one of the classical lignin related building block, vanillin has been proposed as a platform for the preparation of different monomers that could substitute non-sustainable phenol derivatives.46,47
Even more excitingly, resorcinol analogs, which incorporate natural metabolites like dopamine or dihydroxy-L-phenylalanine, have been shown to be an integral part of advanced biomimetic glues48 binding for instance to leather (as known from the traditional art of “tanning leather” with tannic acid derivatives), cellulose fibers, paper and cotton (known from lignin binding in wood), or even to minerals in the presence of water, as in the case of natural mussel adhesives.49 For all these cases, depolymerized lignin phenols seem to be a perfect choice to generate analogs of dopamine or dihydroxy-L-phenylalanine on a high scale, otherwise too difficult to obtain on a sustainable basis. However, it has to be mentioned that beside the lignin depolymerization, biorefinery schemes should include a robust strategy for the demethylation of phenols, considering the high percentage of phenyl methyl ethers in the native lignin structure (Fig. 9).30 Demethylation increases the number of phenolic groups enabling the application of lignin derivatives in redox processes. Solutions to this problem are currently appearing in the literature and are usually based on catalytic hydrodeoxygenation methods.50 As an alternative, building blocks that do not require such demethylation treatments could be identified. In this regard, we should mention the tannin derivative gallic acid, which has been recently described as a promising candidate for the preparation of bio-based epoxy resins.51
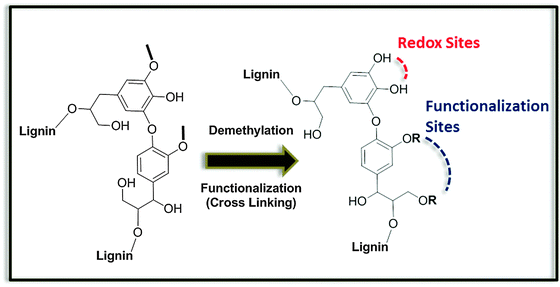 | ||
| Fig. 9 Schematic aspects of the chemical modification of a lignin building block for its incorporation into polymers and materials. | ||
Beyond lignin and cellulose
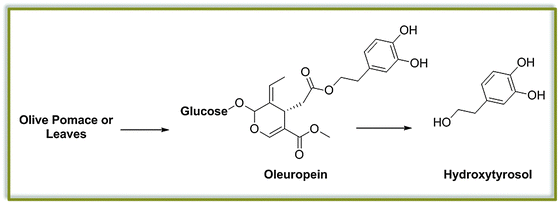
Up to now, we have restricted our attention to molecules which can be generated from the main constituents of lignocellulosic biomass, namely cellulose and lignin. It is worth mentioning that a variety of additional platform molecules and building blocks can be obtained by extraction of plant metabolites contained in the biomass. For instance, nuts as well as seeds and plant leaves (such as the olive kernels and leaves), which are often accumulated as waste streams of agroindustry, contain large amounts of special lignin-like molecules. In case of the olive, for example, the pharmaceutically active and cosmetically relevant hydroxytyrosol, a component of Oleuropein, is a well-known example (Fig. 10).52 In analogy to other lignin-like fragments discussed above (see the case of cardanol),42 this and similar compounds would represent chemicals that are directly suitable for polyurethane chemistry.Analogous transformations could be considered for many families of compounds including tannins,51 polyphenols widespread in the vegetal kingdom, or the well-studied triglycerides, the main component of vegetable oils. The latter, in particular, have been subject of intense study in the context of biorefinery for the production of biofuels, as discussed above. Interestingly, the main components of such abundant vegetal feedstock, namely glycerol and fatty acids, have already found application as platform molecules for the preparation of polymers and new materials. Glycerol derived dimethylacetal dihydroxyacetone carbonate has been successfully reported as building blocks for the preparation of polycarbonates by the group of Waymouth, for instance.53 In parallel, unsaturated fatty acids have been investigated in the last years as starting materials for the production of monomers via cross metathesis reactions. Such building blocks have been already successfully applied for the synthesis of polyesters, polyamides and polyacrylates,54 and their discussion has been already well-covered in the literature.
Finally, it is worth mentioning that besides lignocellulosic biomass marine biomass has also shown significant potential to contribute to the diversification of the products obtainable via biorefinery. Excellent results have been achieved for instance by the group of Maschmeyer in the processing of marine algae for the formation of enriched crude bio-oils via hydrothermal liquefaction.55 The upgrade of such biocrudes, which are generated from biomass lacking lignin and with high proteic contents, may result in the identification of products which expand the spectrum of sustainable building blocks. For more details on this interesting topic, we redirect the reader to more specialized works.
Summary
This tutorial review explored the potential of biorefinery to become a game changer for the field of polymer and material science. In particular, the ability of biorefinery to provide building blocks and platform chemicals which are alternative to the conventional ones obtained via oil refinery was highlighted. Interestingly, such a pool of “biorefined” compounds can be used for the synthesis of polymers and materials with novel interesting properties expanding the field of classical polymers. Increased biocompatibility, lower health risks, improved biodegradation and recyclability, and in some cases expanded thermal and optical properties are only some of the advantages coming with materials prepared on the basis of sustainable building blocks. In this review, we mainly focused on lignocellulosic biomass and summarized the most promising degradative and non degradative processing strategies which are currently utilized to extract or convert the carbohydrate and lignin portion of biomass into useful polymer precursors. In this contest, particular attention was given to the role of hydrothermal and solvothermal strategies that allow for a direct deconstruction of crude biomass into ready-to-use building blocks by very simple procedures. Several examples for the synthesis of low molecular weight building blocks like lactic acid, levulinic acid, or hydroxymethylfurfural were described. Strategies for the application of oligomeric cellulose and lignin during the synthesis of nanocomposites and polymers have also been provided, indicating the arrival of a new generation of glues, binders and coating materials that essentially reinterpret our technological and cultural heritage in modern attire.Acknowledgements
The authors thank Dr Sarah Brosnan for useful comments and corrections.References
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC and references therein.
- L. Caspeta, N. A. A. Buijs and J. Nielsen, Energy Environ. Sci., 2013, 6, 1077–1082 CAS.
- P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2013, 52, 11980–11987 CrossRef CAS PubMed.
- M. Dusselier and B. Sels, in Selective Catalysis for Renewable Feedstocks and Chemicals, ed. K. M. Nicholas, Springer International Publishing, 2014, ch. 540, vol. 353, pp. 85–125, and references therein Search PubMed.
- M. Dusselier, P. Van Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415–1442 CAS and references therein.
- S. Zhang, F. Jin, J. Hu and Z. Huo, Bioresour. Technol., 2011, 102, 1998–2003 CrossRef CAS PubMed.
- C. Sánchez, I. Egüés, A. García, R. Llano-Ponte and J. Labidi, Chem. Eng. J., 2012, 181–182, 655–660 CrossRef PubMed.
- D. Esposito and M. Antonietti, ChemSusChem, 2013, 6, 989–992 CrossRef CAS PubMed.
- F.-F. Wang, C.-L. Liu and W.-S. Dong, Green Chem., 2013, 15, 2091–2095 RSC.
- Y. Wang, W. Deng, B. Wang, Q. Zhang, X. Wan, Z. Tang, Y. Wang, C. Zhu, Z. Cao, G. Wang and H. Wan, Nat. Commun., 2013, 4, 2141 Search PubMed.
- K. M. Z. Hossain, M. S. Hasan, D. Boyd, C. D. Rudd, I. Ahmed and W. Thielemans, Biomacromolecules, 2014, 15, 1498–1506 CrossRef CAS PubMed.
- Y.-L. Chung, J. V. Olsson, R. J. Li, C. W. Frank, R. M. Waymouth, S. L. Billington and E. S. Sattely, ACS Sustainable Chem. Eng., 2013, 1, 1231–1238 CrossRef CAS.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584–595 RSC and references therein.
- M. K. Akkapeddi, Macromolecules, 1979, 12, 546–551 CrossRef CAS.
- L. E. Manzer, Appl. Catal., A, 2004, 272, 249–256 CrossRef CAS PubMed.
- V. Molinari, M. Antonietti and D. Esposito, Catal. Sci. Technol., 2014, 4, 3626–3630 CAS and references therein.
- J. Suenaga, D. M. Sutherlin and J. K. Stille, Macromolecules, 1984, 17, 2913–2916 CrossRef CAS.
- G. Qi, M. Nolan, F. J. Schork and C. W. Jones, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5929–5944 CrossRef CAS PubMed.
- M.-M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103–116 RSC and references therein.
- P. Lanzafame, G. Centi and S. Perathoner, Chem. Soc. Rev., 2014, 43, 7562–7580 RSC and references therein.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed and references therein.
- M. Moliner, Y. Román-Leshkov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6164–6168 CrossRef CAS PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC and references therein.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC.
- G. Chieffi, C. Giordano, M. Antonietti and D. Esposito, J. Mater. Chem. A, 2014, 2, 11591–11596 CAS.
- J. Mitra, X. Zhou and T. Rauchfuss, Green Chem., 2015, 17, 307–313 RSC.
- A. Gandini, Macromolecules, 2008, 41, 9491–9504 CrossRef CAS.
- S. Glatzel, Z. Schnepp and C. Giordano, Angew. Chem., Int. Ed., 2013, 52, 2355–2358 CrossRef CAS PubMed.
- R. T. Olsson, M. A. S. Azizi Samir, G. Salazar Alvarez, L. Belova, V. Strom, L. A. Berglund, O. Ikkala, J. Nogues and U. W. Gedde, Nat. Nanotechnol., 2010, 5, 584–588 CrossRef CAS PubMed.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed and references therein.
- H. Chung and N. R. Washburn, Green Mater., 2013, 1, 137–160 CrossRef CAS and references therein.
- V. Molinari, C. Giordano, M. Antonietti and D. Esposito, J. Am. Chem. Soc., 2014, 136, 1758–1761 CrossRef CAS PubMed.
- J. He, C. Zhao and J. A. Lercher, J. Am. Chem. Soc., 2012, 134, 20768–20775 CrossRef CAS PubMed.
- A. Toledano, L. Serrano, J. Labidi, A. Pineda, A. M. Balu and R. Luque, ChemCatChem, 2013, 5, 977–985 CrossRef CAS PubMed.
- X. Wang and R. Rinaldi, Angew. Chem., Int. Ed., 2013, 52, 11499–11503 CrossRef CAS PubMed.
- P. Ferrini and R. Rinaldi, Angew. Chem., Int. Ed., 2014, 53, 8634–8639 CrossRef CAS PubMed.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC and references therein.
- C. C. Weber, A. F. Masters and T. Maschmeyer, Green Chem., 2013, 15, 2655–2679 RSC and references therein.
- S. Kirchhecker, M. Antonietti and D. Esposito, Green Chem., 2014, 16, 3705–3709 RSC and references therein.
- Y. Qian, Y. Deng, X. Qiu, H. Li and D. Yang, Green Chem., 2014, 16, 2156–2163 RSC.
- G. Milczarek and O. Inganäs, Science, 2012, 335, 1468–1471 CrossRef CAS PubMed.
- C. Voirin, S. Caillol, N. V. Sadavarte, B. V. Tawade, B. Boutevin and P. P. Wadgaonkar, Polym. Chem., 2014, 5, 3142–3162 RSC and references therein.
- C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696–3717 CrossRef CAS PubMed.
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- A. V. Krishnan, P. Stathis, S. F. Permuth, L. Tokes and D. Feldman, Endocrinology, 1993, 132, 2279–2286 CAS.
- M. Fache, E. Darroman, V. Besse, R. Auvergne, S. Caillol and B. Boutevin, Green Chem., 2014, 16, 1987–1998 RSC and references therein.
- M. Fache, R. Auvergne, B. Boutevin and S. Caillol, Eur. Polym. J. DOI:10.1016/j.eurpolymj.2014.10.011.
- H. J. Meredith, C. L. Jenkins and J. J. Wilker, Adv. Funct. Mater., 2014, 24, 3259–3267 CrossRef CAS PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- S. Jin, Z. Xiao, C. Li, X. Chen, L. Wang, J. Xing, W. Li and C. Liang, Catal. Today, 2014, 234, 125–132 CrossRef CAS PubMed.
- C. Aouf, H. Nouailhas, M. Fache, S. Caillol, B. Boutevin and H. Fulcrand, Eur. Polym. J., 2013, 49, 1185–1195 CrossRef CAS PubMed.
- G. Rigane, M. Bouaziz, N. Baccar, S. Abidi, S. Sayadi and R. B. Salem, J. Food Sci., 2012, 77, C1077–C1083 CrossRef CAS PubMed.
- J. Simon, J. V. Olsson, H. Kim, I. F. Tenney and R. M. Waymouth, Macromolecules, 2012, 45, 9275–9281 CrossRef CAS.
- M. A. R. Meier, Green Polymerization Methods, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 9–27 and references therein Search PubMed.
- N. Neveux, A. K. L. Yuen, C. Jazrawi, M. Magnusson, B. S. Haynes, A. F. Masters, A. Montoya, N. A. Paul, T. Maschmeyer and R. de Nys, Bioresour. Technol., 2014, 155, 334–341 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |