Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Acid-catalyzed carboxylic acid esterification and ester hydrolysis mechanism: acylium ion as a sharing active intermediate via a spontaneous trimolecular reaction based on density functional theory calculation and supported by electrospray ionization-mass spectrometry
Hongchang
Shi
*,
Yilei
Wang
and
Ruimao
Hua
Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: shihc@mail.tsinghua.edu.cn; Fax: +86-10-62771149; Tel: +86-10-62772179
First published on 19th November 2015
Abstract
Correction for ‘Acid-catalyzed carboxylic acid esterification and ester hydrolysis mechanism: acylium ion as a sharing active intermediate via a spontaneous trimolecular reaction based on density functional theory calculation and supported by electrospray ionization-mass spectrometry’ by Hongchang Shi et al., Phys. Chem. Chem. Phys., 2015, DOI: 10.1039/c5cp02914g.
In the published article, the activation energy Ea in Table 1 is incorrect. The Ea data actually are the activation energies that the proton (H+) transfers to hydroxyl-oxygen or alkyl-oxygen, not those of generating acylium ions. For example, the C–O bond distance is 1.527 and 1.538 Å in the Fig. 1 (2) and Fig. 2 (2), respectively. The bond has been partly, but not completely disconnected, so the Ea (9.0 and 3.4 kcal mol-1) cannot lead to the formation of the corresponding acylium ions. The Ea that can generate acylium ions must be recalculated in accordance with a new scheme.
Scheme 5 (1) shows that an initial bimolecular system of RCOOR2 + R′OH2+ goes through an effective Lewis collision to complete the hydroxyl-oxygen or alkyl-oxygen protonation. Because the water or alcohol molecule is a good leaving group, this collision caused the C–OR2 bond disconnection, which generates an acylium ion and a water or an alcohol molecule, as shown in Scheme 5 (2)
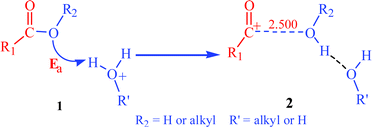 | ||
| Scheme 5 1 is the initial bimolecular system, 2 is the system after an effective Lewis collision. The bond distance is in angstroms. | ||
In the optimization of system 2, the R–C+(O)----O(H)R2 bond distance set here is equal to 2.500 Å. In this spacing, the interaction between the acylium ion and O(H)R2 molecule is slight, that is, the C–OR2 bond is completely disconnected. The 2.500 Å is frozen in the optimization. The E2 of system 2 is higher than the E1 of 1. The elevated energy is the Ea of the hydroxyl-oxygen or alkyl-oxygen protonation: Ea = E2 − E1.
For the protonation of CH3COOH + CH3OH2+ (configuration A), the calculation result is shown as Fig. 1.
| Ea = E2 − E1 = −345.192684 − (−345.218981) = 0.026297 a.u. = 16.5 kcal mol−1. |
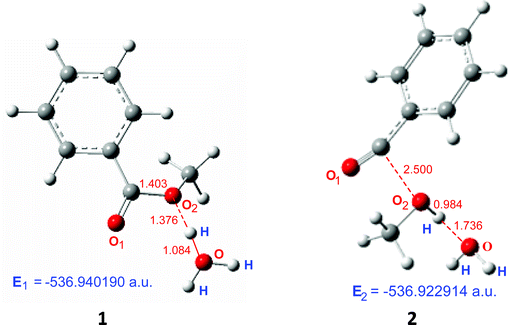
For the alkyl-oxygen protonation of C6H5COOCH3 + H3O+, the calculation result is shown as Fig. 2.
| Ea = E2 − E1 = 0.017276 a.u. = 10.8 kcal mol−1. |
For acetic acid, benzoic acid and their methyl ester, the Ea of their hydroxyl-oxygen and alkyl-oxygen protonations are changed as in the following Table 1.
| System | Config. | E 1/a.u. | E 2/a.u. | E a |
|---|---|---|---|---|
| CH3COOH + CH3OH2+ | A | −345.218981 | −345.192684 | 16.5 |
| B | −345.217477 | −345.190683 | 16.8 | |
| CH3COOCH3 + H3O+ | A | −345.220936 | −345.196416 | 15.4 |
| B | −345.217162 | −345.192777 | 15.3 | |
| C6H5COOH + CH3OH2+ | A | −536.945912 | −536.919611 | 16.5 |
| B | −536.944778 | −536.920010 | 15.5 | |
| C6H5COOCH3 + H3O+ | A | −536.946688 | −536.922924 | 14.9 |
| B | −536.940190 | −536.922914 | 10.8 |
The Ea in Table 1 are much higher than that in the Table 1 of the published article, which is reasonable because the C–OR2 bond is completely disconnected, and this requires more energy.
The Ea values of the hydroxyl-oxygen and alkyl-oxygen protonation in the Abstract, Introduction, Scheme 7 and Scheme 8 should be accordingly changed in accordance with Table 1.
The Table 5 in the published article has been changed as following Table 5. It is also the Ea calculations of hydroxyl-oxygen or alkyl-oxygen protonation.
| Molecular system | Config. | E 1/a.u. | E 2/a.u. | E a/kcal mol−1 |
|---|---|---|---|---|
| (CH3)3C6H2COOH + CH3OH2+ | A | −654.839323 | −654.827446 | 7.4 |
| B | −654.839660 | −654.827455 | 7.7 | |
| (CH3)3C6H2COOCH3 + H3O+ | A | −654.842084 | −654.830307 | 7.4 |
| B | −654.837079 | −654.829814 | 4.6 |
For the Ea calculation of carbonyl-oxygen protonation in the published article, the calculation scheme and result (Table 2) are correct because the protonation is a simple proton transfer.
The change mentioned above not only does not affect the conclusions in the published article, but also helps to support these conclusions.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Owner Societies 2015 |