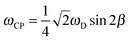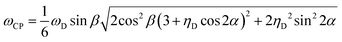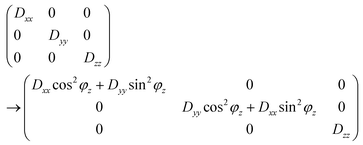Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Analysis of local molecular motions of aromatic sidechains in proteins by 2D and 3D fast MAS NMR spectroscopy and quantum mechanical calculations†
Piotr
Paluch
a,
Tomasz
Pawlak
 a,
Agata
Jeziorna
a,
Julien
Trébosc
b,
Guangjin
Hou
c,
Alexander J.
Vega
c,
Jean-Paul
Amoureux
bd,
Martin
Dracinsky
*e,
Tatyana
Polenova
*c and
Marek J.
Potrzebowski
*a
a,
Agata
Jeziorna
a,
Julien
Trébosc
b,
Guangjin
Hou
c,
Alexander J.
Vega
c,
Jean-Paul
Amoureux
bd,
Martin
Dracinsky
*e,
Tatyana
Polenova
*c and
Marek J.
Potrzebowski
*a
aPolish Academy of Sciences, Centre of Molecular and Macromolecular Studies, Sienkiewicza 112, PL-90-363 Łodz, Poland. E-mail: marekpot@cbmm.lodz.pl
bUnit of Catalysis and Chemistry of Solids (UCCS), CNRS-8181, University Lille North of France, 59652 Villeneuve d'Ascq, France
cDepartment of Chemistry and Biochemistry, University of Delaware, Newark, Delaware, USA. E-mail: tpolenov@udel.edu
dPhysics Department & Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai 200062, China
eInstitute of Organic Chemistry and Biochemistry, AS CR, Flemingovo nam. 2, Prague, Czech Republic. E-mail: dracinsky@uochb.cas.cz
First published on 25th September 2015
Abstract
We report a new multidimensional magic angle spinning NMR methodology, which provides an accurate and detailed probe of molecular motions occurring on timescales of nano- to microseconds, in sidechains of proteins. The approach is based on a 3D CPVC-RFDR correlation experiment recorded under fast MAS conditions (νR = 62 kHz), where 13C–1H CPVC dipolar lineshapes are recorded in a chemical shift resolved manner. The power of the technique is demonstrated in model tripeptide Tyr–(D)Ala–Phe and two nanocrystalline proteins, GB1 and LC8. We demonstrate that, through numerical simulations of dipolar lineshapes of aromatic sidechains, their detailed dynamic profile, i.e., the motional modes, is obtained. In GB1 and LC8 the results unequivocally indicate that a number of aromatic residues are dynamic, and using quantum mechanical calculations, we correlate the molecular motions of aromatic groups to their local environment in the crystal lattice. The approach presented here is general and can be readily extended to other biological systems.
Introduction
In recent years solid-state Nuclear Magnetic Resonance (NMR) spectroscopy has become one of the most powerful techniques in structural studies of condensed matter, in particular for synthetic polymers and biological systems.1 A number of highly exciting applications have been reported for the latter group of samples including characterization of proteins and other biopolymers that are inaccessible to X-ray crystallography and solution-state NMR spectroscopy.2 Today the palette of available methodological NMR approaches revealing general and specific information about local geometry, intra- and inter-molecular interactions, and higher order structure is very rich.3 The full assignment of resonances and determination of molecular structure can be done by employing different multi-dimensional solid-state NMR protocols. This issue was exhaustively discussed in recent review articles by us and by others.4–7To understand in detail the biological functions of proteins, knowledge of the molecular dynamics is crucial. It is well known that protein motions occurring on the pico- to millisecond time scales play important functional roles, and that dynamics modulates interactions between physiological partners. The unique feature of NMR spectroscopy is the possibility of the analysis of the dynamics of biomolecules at atomic resolution.8 Once signals are assigned, measurements can be made of relaxation rates, residual anisotropic interactions (dipolar, quadrupolar and chemical shift), relaxation dispersion profiles, magnetization- and hydrogen exchange rates; all yielding site-specific information about dynamics. However, one of the biggest achievements in the field of solid-state NMR spectroscopy is the introduction of fast magic angle spinning (MAS) probes presently delivering rotation frequencies higher than νR = 110 kHz.9 These very fast rates have opened new possibilities in studies of proteins.10 On the other hand, anisotropic tensorial interactions, e.g. dipolar and chemical shift, which are the source of crucial information about the structure and dynamics of natural products are eliminated or averaged to their isotropic values due to very fast sample spinning. Hence, a number of techniques have been developed during recent years to reintroduce those interactions and a large variety of pulse schemes have been published.11–15 Recoupling of anisotropic spin interactions is achieved by application of pulse sequences that are often based on symmetry rules introduced by Levitt.16 These sequences are called CNnν and RNnν, where the symbols N, n, and ν are small symmetry integer numbers that depend on the rotation properties of the spin angular moment during the rotor-synchronized train of radio frequency (RF) pulses. In this strategy the relationship between spins, space, rotation and radiofrequency pulses is used to amplify the desired and suppress the unwanted interactions. The recently published RN-CSA symmetry schemes are suitable for CSA recoupling under fast spinning speed (νR ≥ 40 kHz).17
Symmetry-based approaches were also employed by several research groups to study hetero-nuclear dipolar couplings in proteins.18 These sequences are highly effective in suppressing 1H–1H homonuclear dipolar couplings, which make them suitable in fully protonated systems. In these symmetry-based experiments the RF pulses have to be synchronized with the sample rotation, which is not always easy to achieve in case of very fast sample spinning (e.g. νR ≥ 100 kHz) due to the proportionality between the required RF fields and νR, and also to the transients which are not negligible with such small rotor periods.
In a recent article employing small molecules as model samples, we have shown that a simple Cross Polarization with Variable Contact (CPVC) experiment performed under fast MAS conditions is free from the discussed supra-limitations and provides very accurate values of hetero-nuclear (13C–1H, 15N–1H) dipolar couplings, D, which reflect the inter-atomic distances and/or molecular motions.19 We have also shown that CPVC methods are very robust with respect to RF-inhomogeneity, CSAs and offsets. Recent papers, from us and others, have fully confirmed the applicability of this approach.20–22
In the current work we show that this methodology is a starting point for more advanced multi-dimensional techniques, which can be applied to study large biological systems. The power of this methodology is illustrated on 13C enriched models: tripeptide Tyr–(D)Ala–Phe and two proteins, GB1 and LC8. We demonstrate that using our approach, we can site-specifically assign the side-chain resonances of the aromatic residues, which is typically a challenge in proteins. With these assignments in hand, we infer the molecular dynamics of the aromatic residues. To do so, we employ an average Hamiltonian approach to simulate the dipolar lineshapes. This approach permits unequivocal identification of the motional modes of the aromatic sidechains undergoing nano- to microsecond time scale motions. Furthermore, by combining our experimental NMR results with theoretical calculations, we show that the motional modes correlate with the location of a particular amino acid in the context of the protein structure. The methodology reported here is general and readily extendable to other biological systems of interest, including non-crystalline proteins, protein assemblies, and other bio-macromolecules. At this stage of hardware development, the 3D CPVC approach (as most of multi-dimensional solid-state NMR techniques) can only be applied to isotopically enriched samples. However, introducing new NMR concepts and techniques (e.g. very fast DNP CP/MAS) may shortly extend the applicability of the method to unlabeled samples.
Experimental
Materials
U–13C Tyr–(D)Ala–Phe (YAF) tripeptide was synthesized using labeled amino acids purchased from Sigma-Aldrich (D-alanine-13C3-99%) and Cambridge Isotope Laboratories, Inc. (L-tyrosine-13C9-99%, 15N-99% and L-phenylalanine-13C9-99%, 15N-99%). Its synthesis was carried out in solution according to procedure reported in our previous paper.23U–13C,15N-GB1 was bought from Giotto Biototech Company as “ready-to-use” sample packed in 1.3 mm rotor. Dynein light chain 8 (LC8) protein was prepared according to the protocol reported by us previously.24 3 mg of U–13C,15N-LC8 prepared by controlled precipitation from polyethylene glycol were packed into a 1.3 mm rotor and used for subsequent MAS NMR experiments.
NMR spectroscopy
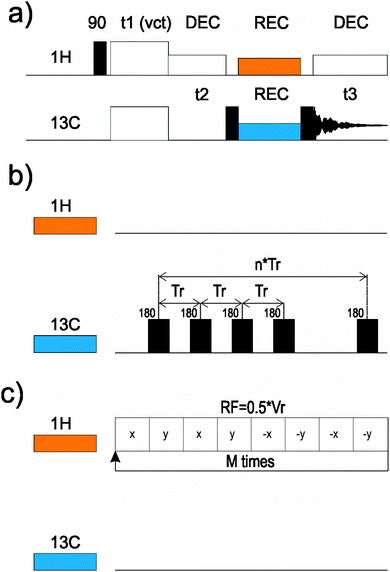
All experiments were measured on a Bruker Avance III spectrometer equipped with a 1.3 mm 1H/31P–15N probehead, spinning at νR = 62 kHz, and operated at frequencies of 600.1 and 150.9 MHz for 1H and 13C, respectively. In all cases the π/2 pulse durations were of 2.5 μs for 1H and 1.5 μs for 13C. We used a low power (RF = 10 kHz) swftppm decoupling25,26 during acquisition.2D 13C–13C RFDR-8m41 correlations27 were measured with: (i) 166 kHz RF; (ii) mixing times of 8 or 16 ms; (iii) a matrix size of 2560 × 1024 points; and (iv) a spectral width of 220 × 220 ppm; giving acquisition times of 38.4 and 15.4 ms in direct and indirect 13C dimensions. Experiments were measured with ramp CP from 70 to 100% on the 1H channel, with RF ≈ 100 kHz. We collected 40 scans for proteins and 8 scans for YAF.
3D CPVC-RFDR experiments used 8 ms mixing time and 166 kHz RF for RFDR-8m41. For proteins, we measured 16 scans, matrix sizes of 2560 × 40 × 480 points, with spectral widths of 220 ppm × 60 kHz × 220 ppm giving acquisition times of 38.4 × 0.667 × 7.2 ms in 13C direct, dipolar and 13C indirect dimensions. For YAF, the 3D spectrum was measured with 8 scans, a matrix size of 1782 × 48 × 320 points, and spectral widths of 197 ppm × 60 kHz × 197 ppm giving acquisition times of 38.4 × 0.667 × 10.8 ms in 13C direct, dipolar and 13C indirect dimensions.
In all 3D CPVC-RFDR experiments, Hartmann–Hahn matching conditions were precisely calibrated on the sample by changing 1H RF near to 104 kHz, while 13C RF was kept constant at 166 kHz.
Simulations of CPVC dipolar lineshapes
The simulations of CPVC dipolar lineshapes were performed in the time-domain mode. Based on the following considerations we could do it by application of the simple algebraic expressions quoted below instead of having to go through time-consuming full quantum mechanical calculations.Under MAS, the CP magnetization transfer from the 1H spin polarization to the to-be-detected 13C spin polarization can be performed either in zero-quantum (ZQ) or double-quantum (DQ) mode. ZQ-CP is optimized under a so-called Hartmann–Hahn-sideband condition requiring that the difference of the RF amplitudes applied to the two nuclei is equal to once or twice the spinning speed.28 DQ-CP requires that the RF-amplitude sum equals once or twice the spinning speed.29
The CPVC method uses a sum or difference of RF-fields equal to the spinning rate as it reintroduces the maximum of dipolar interactions, hence leading to the maximum splitting of Δ = D/√2 in the case of static moieties. Moreover, it has been shown that ZQ-CP transfers perform better than DQ-CP ones, due to their larger possible RF fields.
In our recent publication20 it was reported that spectra obtained under standard experimental conditions closely follow the results predicted under the assumption of ideal Hartmann–Hahn matching. It was also shown that non-ideal conditions, such as RF inhomogeneity, CSAs, resonance offsets, and homo-nuclear proton interactions, have very limited effects on the CPVC lineshape. Globally, the distortions are mainly observed in the region near zero frequency, leading us to exclude the central portion of the spectrum when performing the lineshape fittings. Another effect is a small increase of the apparent D value (less than 1 kHz), which is inconsequential and is neglected in our analysis.
These observations assured us that the current CPVC spectra can be analyzed in terms of the spin-evolution formalism of the idealized first-sideband Hartmann–Hahn situation, according to the intended RF-amplitude configuration. For a single 13C–1H spin pair, the 13C signal after a CP contact time, τ, is given by
| S(τ) = 1 − cos(ωCPτ) |
In the case of an immobile spin pair, the ωCP frequency is given by
When the CH bond undergoes very fast orientational motions, the size of the effective dipolar tensor is reduced to one having principal components Dxx, Dyy, Dzz, thereby possibly losing its axial symmetry.32 In such a case, we identify the largest of the absolute values of the three components as ‘the’ DCC, represented by ωD and the difference of the two other components divided by ωD, as the asymmetry parameter ηD.
The ωCP frequency is then given by
We simulated the CPVC signal in a home-written Fortran-95 program by summation of S(τ) over 2000 powder-averaged tensor orientations. This signal was then shifted by the experimental dead time, followed by Lorentzian apodization, and FT. Since the term 1 in the expression for S(τ) creates the large time-independent signal component that in the experimental spectra is eliminated by appropriate baseline correction prior to the FT, we simulated S(τ) simply as cos(ωCPτ). The experimental CPVC spectra were obtained by FT of the time-domain signals extracted from the original 3D datasets for each well-resolved aromatic peak. The best-fit parameters for the dipolar coupling constant (ωD), dipolar asymmetry parameter (ηD), and line broadening parameter (LB) were obtained by simplex least-squares minimization. Since experimental artifacts such as RF inhomogeneity and chemical-shift offsets often cause lineshape distortions in the central region, we disregard those regions in the fitting procedure. The width of this region is determined by the particular lineshape features in each individual case. We note that dipolar lineshapes corresponding to the desired carbon atom can be obtained using any cross peak with the frequency corresponding to this atom in the indirect or direct 13C dimension, and, as discussed in the Results section, the dipolar parameters derived from the different cross peaks corresponding to the same carbon atom are consistent to within the experimental error.
Quantum mechanical calculations
The energy of the infinite crystal of YAF tripeptide was calculated by the CASTEP program,33 which is a DFT-based code. Electron-correlation effects were modeled using the generalized gradient approximation of Perdew, Burke, and Ernzerhof.34 The calculations were performed with ‘on-the-fly’ pseudopotentials, and a planewave cutoff energy of 600 eV with integrals taken over the Brillouin zone using a Monkhorst–Pack35 grid with a minimum k-point sampling of 0.05 Å−1. The atomic coordinates were derived from the Cambridge Crystallographic Database36 (refcode IWANID23). The positions of all atoms were optimized at the same computational level prior to the aromatic ring rotations. The energy dependence on the PHE/TYR rotation was calculated on a 2 × 2 × 1 supercell so that the rotation of one phenyl ring did not result in the same rotation of the neighboring rings under the periodic conditions. The energy calculations were performed with and without the dispersion correction.37For the cluster calculations, an aromatic ring was selected, then the selection was expanded to all atoms closer than 5 Å (including the atoms of the periodic images in the neighboring unit cells), and then the selection was expanded to include complete amino acid residues. Free C-termini amino acids were ended with amide (CONH2) groups. The energy dependence on the phenyl ring rotation around the Cβ–Cγ bond was calculated at Hartree–Fock level using a standard 6-31G* basis set within the Gaussian-09 program package.38 The orientation of the OH group of TYR residues was kept fixed so that the hydrogen bond network of the crystal was not disturbed.
The same procedure for cluster constructions was used for the GB1 and LC8 proteins. Crystal structures 2QMT and 3DVT from Protein Data Bank39 served as the starting structures.40 Hydrogen atoms were added manually and their positions were optimized at the same computational level prior to the ring rotations and energy calculations.
Results and discussion
NMR spectroscopy
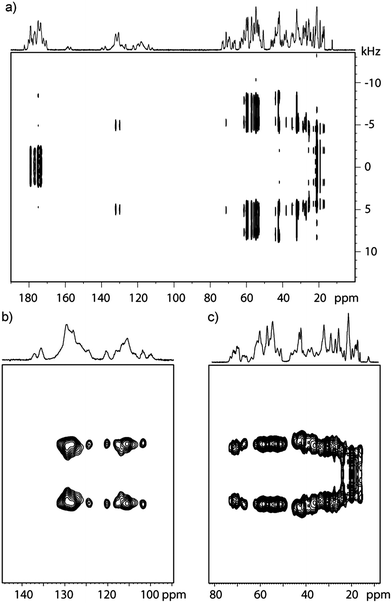
The 2D CPVC spectra of GB1 recorded with the spinning frequency of ωR = 62 kHz are shown in Fig. 1a (full spectrum) and Fig. 1b and c (expansions around aromatic and aliphatic regions). The appropriate spectra for LC8 protein are attached in the ESI† (Fig. S2).
On the first glance, the spectral resolution appears to be sufficiently high as to permit assignment of almost all 13C resonances. However, upon further inspection of the spectrum it becomes clear that we can only determine coarse values of dipolar splittings in the aromatic and aliphatic regions. Inspection of F1 slices indicates that peaks corresponding to the individual amino acid residues overlap, precluding extraction of site-specific information concerning the different motional modes. This overlap is further exacerbated in LC8, due to the larger number of residues. As one can see in Fig. 1b and c, both aromatic and aliphatic residues are overcrowded, and so the dipolar lineshapes for the individual carbon sites overlap.
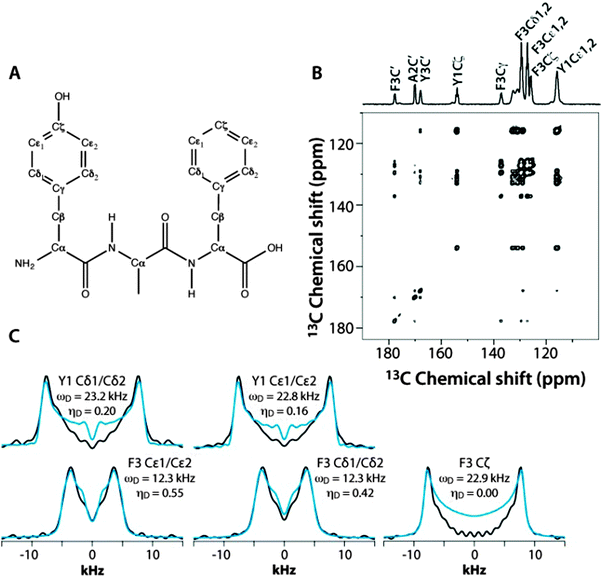
The applicability and accuracy of the CPVC-RFDR method were first validated using a simple model compound, the U–13C labeled YAF tripeptide. This sample is an excellent test case due to the well-defined and distinct dynamics of the two aromatic residues.41 Indeed, the phenyl ring of Tyr is rigid, while that of Phe is mobile and undergoes π jumps at the picosecond rate at room temperature.41
These fast (on the NMR time scale) motions are expected to give rise to partial averaging of the Phe C–H dipolar tensors associated with the Cδ1, Cδ2 and Cε1, Cε2 carbon atoms (the two pairs of resonances overlap), resulting in a reduced dipolar frequency of ωD = 11.25 kHz, which is ca. half of the rigid-limit value of 22.5 kHz, and a non-zero dipolar asymmetry parameter, ηD.
Fig. 3 shows the 2D RFDR plane from the 3D CPVC-RFDR spectrum of the YAF peptide and five selected CPVC lineshapes extracted from the 3D spectrum. The dipolar interaction parameters, ωD and ηD, extracted from the AHT-based simulations of the CPVC lineshapes in the time domain, are presented in the Fig. 3 and in Table S2 of the ESI.† The dipolar parameters for the Tyr and Phe residues are very different; the reduced dipolar frequency and non-zero dipolar asymmetry parameter for Phe indicate the presence of dynamics. The reduction of the dipolar splitting due to the molecular motions of phenyl rings in organic polymers and biological systems is well known and was discussed in prior reports including our previous paper.41–44 The Cδ1, Cδ2 and Cε1, Cε2 carbons of the Phe residue exhibit motionally reduced dipolar interaction parameters: ωD = 12.3/12.2 kHz and ηD = 0.42/0.45. These dipolar parameters suggest composite motions consisting of the π-jumps and additional motional modes, vide infra. In contrast, the Cζ carbon, whose dipolar tensor does not experience such motional averaging due to symmetry (Fig. 3A), exhibits dipolar interaction parameters very close to the rigid limit values, ωD = 22.9 kHz and ηD = 0.0. As discussed later in the text, this finding provides an important clue in defining the motional models for this residue. For the tyrosine residue, the dipolar interaction parameters for Cδ1, Cδ2 and Cε1, Cε2 carbons indicate an almost rigid phenyl ring, with ωD = 23.2/22.8 kHz and ηD = 0.20/0.16, which is close (but not identical) to the rigid-limit values of 23 kHz and 0.0, respectively. That tyrosine's Cδ1, Cδ2 and Cε1, Cε2 as well as the phenylalanine's Cζ show slightly depressed dipolar couplings and non-zero dipolar asymmetry parameters (the deviations are well outside of the experimental errors) suggest the presence of some mobility in the ns–μs regime, likely small-amplitude out-of-plane wobbling motions.
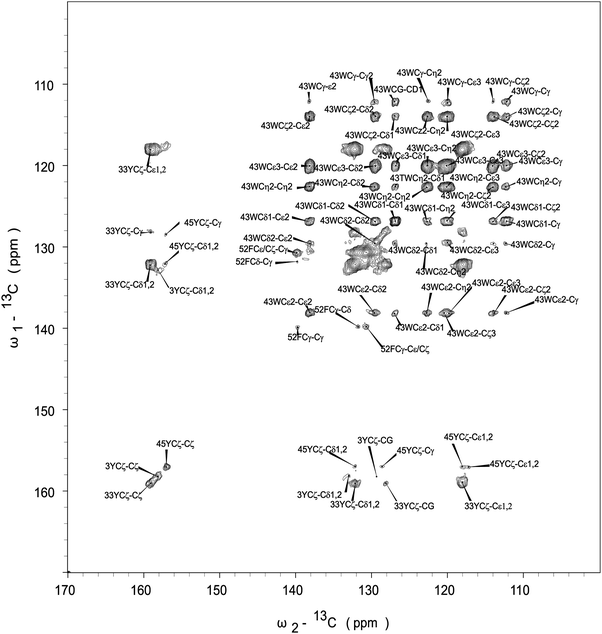
The assignment of Phe-52 is also straightforward. The chemical shift of F52Cγ is 139.9 ppm, and it correlates with its Cε/Cζ whose shift is 131.0 ppm. Other correlations for Phe appear as intense overlapping signals found in the region of 130–132 ppm, and we could not unequivocally assign Phe-30 resonances. The assignment of resonances for Trp-43 is unambiguous. The correlation peaks between quaternary C43ε carbon (δ = 138.2 ppm) and other carbons are clearly present.
Fig. 5 shows the CPVC dipolar lineshapes for the aromatic carbons in GB1 extracted from the 3D CPVC-RFDR spectrum. Indeed, the dipolar parameters were obtained as best fits by the AHT-based simulations of the lineshapes, and are summarized in Table S2 of the ESI.† The results indicate that W43 does not undergo any significant dynamics. The dipolar interaction parameters extracted for all carbon atoms that have attached protons are very close to the rigid limit values, ωD = 23 kHz and ηD = 0. The observed deviations from these parameters for GB1 are attributed to experimental errors, which are larger than in the YAF tripeptide because of lower signal-to-noise ratio in the spectra. The fact that the CPVC lineshapes for W43 and the corresponding dipolar interaction parameters extracted from 14 different diagonal and cross peaks are consistent is an important validation of the robustness of the CPVC-RFDR experiment. Similar to W43, F52 exhibits nearly rigid behavior with ωD = 21.9 ± 0.1 kHz and ηD = 0.03 ± 0.03.
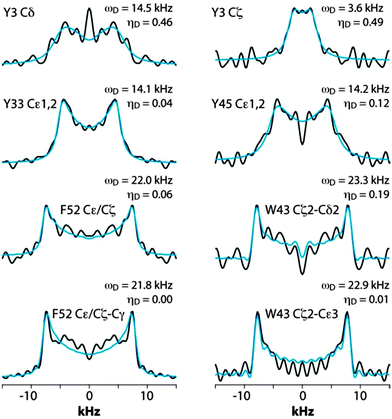 | ||
| Fig. 5 CPVC dipolar lineshapes for representative aromatic carbons in GB1 extracted from the 3D CPVC-RFDR spectra. The experimental and best-fit simulated lineshapes are shown as black and blue lines, respectively. The dipolar interaction parameters are indicated next to each lineshape and summarized in Table S2 (ESI†). | ||
In contrast to W43 and F52, the tyrosine residues are dynamic. For Y33, the values for Cδ and Cε carbons, averaged over several cross peaks, are ωD = 14.0 ± 0.2 kHz, and ηD = 0.03 ± 0.2. For Y45, they are ωD = 14.2 kHz, and ηD = 0.12. These parameters are not consistent with simple fast π-jumps around the 1–4 axis where ωD is expected to be 14.0 kHz and ηD = 0.6 (vide infra). The nearly axial dipolar interaction suggests that the aromatic ring undergoes a motion more complex than the π rotation around the Cγ–Cζ axis. Similarly, in Y3, more complex motions than the π rotations are observed: ωD = 14.5 kHz and ηD = 0.46. We will discuss the possible nature of such complex motions later in the report.
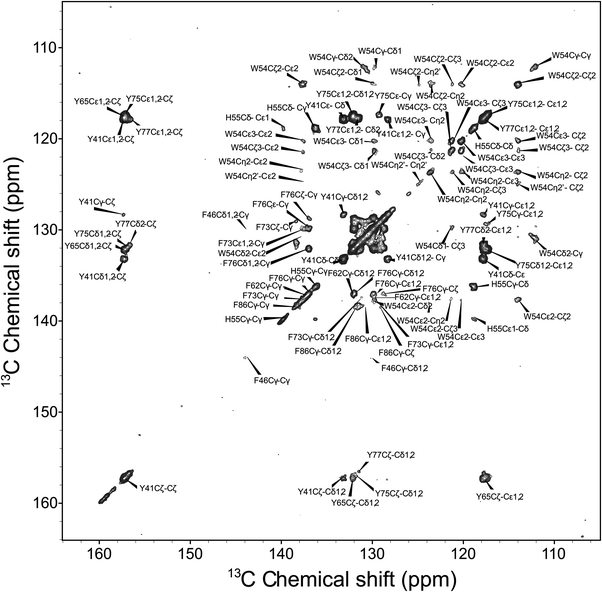
We next discuss the analysis of the molecular dynamics of the aromatic residues in LC8. Fig. 6 shows the expansion around the aromatic region of the 13C–13C 2D RFDR correlation spectrum. The full spectrum is displayed in Fig. S1 of the ESI.† LC8 contains six tyrosines (Y32, Y41, Y50, Y65, Y75, and Y77), five phenylalanines (F46, F62, F73, F76, and F86), three histidines (H55, H68, and H72), and one tryptophan (W54). We have reported the residue-specific assignments of the aliphatic and carbonyl carbons of LC8 previously.51,52 However, in those earlier data sets aromatic sidechain assignments could not be obtained. As shown in Fig. 6, the quality of the 2D RFDR spectrum is such that the assignments of the majority of the aromatic sidechains were readily accomplished. The chemical shifts are summarized in Table S1 of the ESI.† With these assignments in hand, the analysis of the dipolar lineshapes from the 3D CPVC-RFDR spectra was straightforward for many of the aromatic residues in LC8. Fig. 7 shows the experimental and simulated dipolar lineshapes for representative residues that yield resolved cross peaks. The corresponding dipolar parameters are summarized in Table S2 of the ESI.†
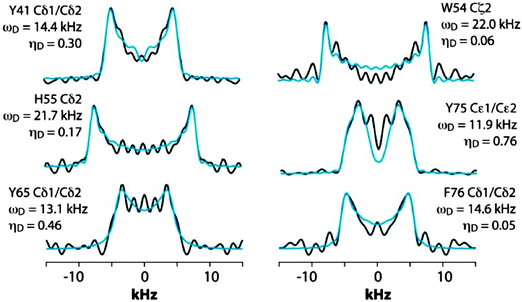 | ||
| Fig. 7 CPVC dipolar lineshapes for representative aromatic carbons in LC8 extracted from the 3D CPVC-RFDR spectrum. The experimental and best-fit simulated lineshapes are shown as black and blue lines, respectively. The dipolar interaction parameters are indicated next to each lineshape and summarized in Table S2 (ESI†). | ||
The only tryptophan residue in LC8, W54, exhibits ωD = 21.8–22.0 kHz and ηD = 0.06–0.20. These values indicate that W54 is nearly rigid in LC8, which is consistent with the fact that it is located in a β-sheet and packed against several other aromatic residues: F46, Y50, and H70. Similarly, in H55 the field values of ωD = 21.9 ± 0.2 kHz and ηD = 0.11 ± 0.05 indicate the lack of large-amplitude motions of the aromatic ring. This is not very surprising given the fact that H55 (i) belongs to a β-sheet, (ii) comprises the interface of the LC8 homodimer, and (iii) is packed against three aromatic residues: F86 located in the same LC8 monomer as well as F86 and H55 of the neighboring molecule.
F76, the only phenylalanine with non-overlapping signals whose dipolar lineshapes we have been able to analyze, is dynamic. The dipolar parameters recorded for Cδ1/Cδ2, ωD = 14.6 kHz and ηD = 0.05, indicate complex motions similar to those related to our observations for Y33 of GB1, and the model that will be discussed in the next section apply.
For tyrosines, spectral overlap precludes analysis of dipolar lineshapes for Y32 and Y50. For the other four tyrosines, Y41, Y65, Y75, and Y77, the results unequivocally indicate the presence of dynamics. The dipolar parameters for all tyrosines suggest complex motional modes, the models for which are discussed in the next section.
Under such circumstances, it is not possible to determine the dipolar interaction parameters from the peak splittings alone, as has been suggested in prior studies,19 and lineshape simulations are required. As discussed earlier in the text, accurate dipolar interaction parameters are derived by simulations of CPVC spectra using the average Hamiltonian approach.
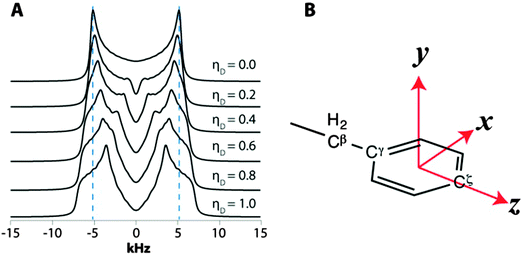
Interestingly, the dipolar interaction parameters for many carbon sites in our experiments are indicative of motions more complex than π-jumps around the Cγ–Cζ axis of the aromatic ring, where ωD = 14.0 kHz and ηD = 0.6. To describe the possible motional models giving rise to such dipolar interaction parameters, we define an xyz axis system in the aromatic ring illustrated in Fig. 8B: z – along the Cγ–Cζ axis; x – perpendicular to z in the aromatic plane; and y – perpendicular to the aromatic plane.
The models are kept as simple as possible by restricting them to rapid jumps of the dipolar tensor between two equally occupied orientations that are rotated away over angles ±ϕi from an unoccupied central orientation, whereby the rotation axis is parallel to one of the principal tensor axes (i = x, y or z). If the rotation is about the z axis, then the conversion of the dipolar tensor from the static central situation to the average of the rotated situations is given by
A similar averaging is obtained for rotation about x or y axes.
The main motional process of the tyrosine ring is 180° flips about the z-axis. For the four carbons that are not along the z-axis, this can be viewed as rotational jumps about a central position consisting of an axially symmetric dipolar tensor with its main axis along z, making ϕy = ±60° jumps about y. From the CPVC spectra of immobile aromatic sites we know that the static dipolar frequency is ωD ≈ 22.4 kHz. Hence, the ring flips lead to the following averaging of the tensor components (Dxx, Dyy, Dzz in kHz): (−11.2, −11.2, 22.4) → (14.0, −11.2, −2.8), i.e. ωD = 14.0 kHz, ηD = 0.6.
These parameters deviate from the experimentally observed values by more than the experimental error.
Moreover, we cannot ascribe the deviations to π-ring flips that occur at correlation times between the extreme-narrowing limit of 10−7 s and the rigid limit of 10−4 s, thereby creating interesting transition lineshapes. Indeed, we have performed such simulations (not shown), and not only do the lineshapes poorly fit the experiment, but these intermediate correlation times also give rise to very short T1ρ relaxation times that produce huge negative center peaks in the CPVC spectra. Those are not observed in our experimental data.
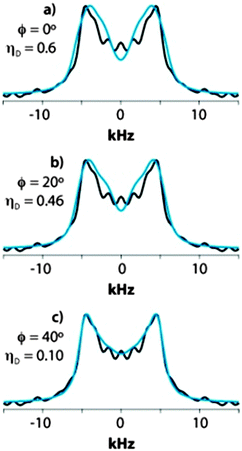
In the case of Y33 (GB1) and F76 (LC8), ωD value found by the fit equals that of the two-site flip model, but ηD is nearly zero instead of the 0.6 predicated by the model. This suggests that there is an additional rotation about the x-axis (the orientation where the dipolar component is not affected). Such up-and-down flaps of varying angles give the following average dipolar tensors:
| ϕx = 10° → (14.0, −11.0, −3.1), i.e., ωD = 14.0 kHz, ηD = 0.56 |
| ϕx = 20° → (14.0, −10.2, −3.8), i.e., ωD = 14.0 kHz, ηD = 0.46 |
| ϕx = 30° → (14.0, −9.1, −4.9), i.e., ωD = 14.0 kHz, ηD = 0.30 |
| ϕx = 40° → (14.0, −6.3, −7.7), i.e., ωD = 14.0 kHz, ηD = 0.10 |
A particular observation is that the peak splitting increases from 8.0 to 8.9 kHz when the rotation angle ϕx increases from 0 to 40°. This contradicts the conventional assumption that a smaller splitting implies a smaller “order parameter”, i.e., a larger amplitude of molecular motion. This result, counterintuitive on the first glance, underscores the importance of fitting the lineshapes for deriving accurate dipolar tensor parameters.
The above result suggests that, in addition to the 180° flips, the phenyl rings in Y33 (GB1) and F76 (LC8) also flap up and down over an angle of about 2 × 40°. This could be related to trans–gauche-type transformations at Cβ and be accommodated by bond-angle changes of the OH group and its hydrogen-bonding. We note that the relatively large line broadening parameter required to fit the spectra may either be a relaxation effect caused by these large-amplitude motions, or a distribution of the ϕx angles.
For F3 residue in YAF and for Y41, Y65, Y75, and Y77 in LC8, the CPVC lineshapes deviate in a different way from a simple π-jump model. In this case ηD is close to the predicted 0.6 but ωD is reduced to 10.4–12.4 kHz. This can be reproduced by applying one additional small-angle two-site jump. In the case of F3 in YAF it is clear that the second rotation has to be about the z-axis, because the Cζ carbon exhibits rigid-limit dipolar interaction values, which would be inconsistent with rotations about either the x- or the y-axis. Incorporating the second rotation about the z-axis into our motional model for the YAF's F3 residue, we obtain the best fit to the experimental results when the ring undergoes additional rotations by ±15° about the z-axis. So, we may conclude that when the phenyl ring makes π flips about the z-axis it has a wide range of possible orientations around its energy minimum, ca. 15° to each side, in agreement with the very shallow minimum around 0 and 180° found in the quantum mechanical calculations, vide infra.
For tyrosine residues in LC8, we cannot constrain the motional model in a similar way because Cζ carbons do not carry a proton. In the absence of such constraints, more than one motional profile is consistent with the experimental data.
An alternative computational approach for the assessment of the side-chain motions could be molecular dynamics simulations with their tremendous advances in performance and applicability in recent years.53–55 However, the size of the observed dipolar couplings suggests that micro- to millisecond simulation times would be required for a proper capturing of the molecular motions, which is still beyond the capability of conventional simulation techniques. The below described calculations of rotational barriers thus represent a computationally cheap and widely applicable approach for a qualitative link between observed NMR data and side-chain mobility.
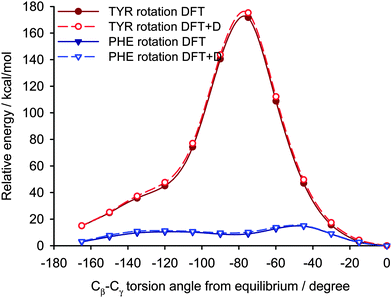
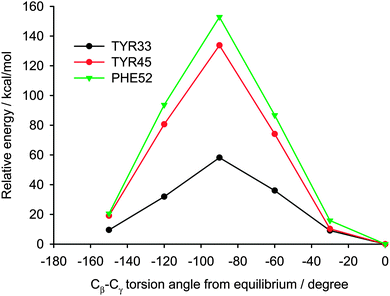
In explaining the differences in the C–H dipolar splittings of aromatic groups in the crystal lattice we assumed that even subtle distinctions in the local environment will influence the energy barrier of free rotation around the 1–4 axis of phenyl rings. The aim of the study reported in this section is an analysis of energetic parameters employing the theoretical approach and their correlation with experimental values of the DCC. For testing the methodology we have used X-ray data for YAF peptide (refcode IWANID) constructing the appropriate cluster surrounding each phenyl residue. Fig. 10 shows the crystal lattice motif with clearly visible phenyl groups of Phe inside a cage. The aromatic residue has enough space for free rotation. The molecular motion of phenyl ring of Tyr is more restricted due to presence of the neighboring methyl groups and hydrogen bonds.
 | ||
| Fig. 10 Visualization of the YAF supramolecular array. The coordinates are taken from ref. 23. | ||
Two different computational approaches were used to estimate the mobility of the aromatic rings in YAF peptide. First, crystal calculations with fully periodic boundary conditions of the optimized YAF structure and of that with changed orientation of Phe or Tyr aromatic rings were used to estimate the barrier of aromatic ring rotation. Significant differences between the barriers of rotation of aromatic rings of Tyr and Phe are clearly visible in Fig. 11.
Indeed, the rotation barrier of Phe is much lower than that of Tyr, which is in agreement with previous observations of significant differences in the mobility of these phenyl rings in the YAF peptide.23,41 The rotation barriers in a real system will be probably slightly lower than the calculated ones for both rings because the surrounding molecules can adapt to the transition geometry.
The second approach of the rotation barrier estimation consists in energy calculations for a cluster of molecules cut out of the infinite crystal structure with the studied aromatic ring in the center of the cluster. This approach is commonly used for prediction of NMR parameters of crystalline samples.56 The Hartree–Fock energies calculated for the cluster models of YAF peptide overestimate the rotation barriers slightly (Fig. 12), but for a qualitative insight into the mobility of aromatic residues within a polypeptide structure these calculations are sufficient. The advantage of the cluster approach is its applicability to protein structures where the calculations with periodic boundary conditions would be prohibitively computationally expensive. The comparison with the energies calculated with the periodic approach validates the cluster model, which is then applied for the GB1 protein.
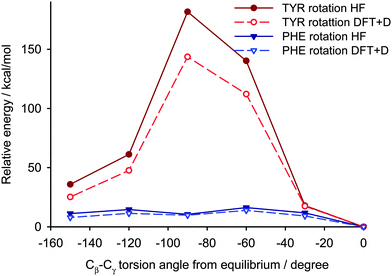 | ||
| Fig. 12 The comparison of cluster calculations (at the Hartree–Fock level) with DFT periodic boundary calculations of rotation barriers of aromatic rings in the YAF peptide. | ||
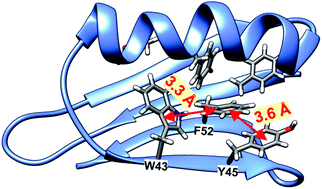
Analyzing the surrounding of aromatic residues we have found that the ring of Tyr33 faces the area between a helix and a sheet (Fig. 13). In this position the phenyl ring has enough space for molecular motion, which is confirmed by the cluster calculations. The energy barrier for Tyr33 rotation was found to be less than half of that for Tyr45 and Phe52 (Fig. 14). Tyr45 is located in the neighborhood of Phe52. The distance between the aromatic groups is below 4 Å and favors the π–π contacts. The rings under discussion are not aligned in one plane but form a small angle; and it is known from literature that a small offset strengthens such interactions.57
Thus we can assume that molecular motions of Tyr45 and Phe52 are limited to small-amplitude wobbling around the 1–4 axis. Phe52 has an additional T-shape short-range contact (3.3 Å) with the adjacent tryptophan residue (Fig. 13). The order of the calculated barrier heights agrees well with the order of experimental dipolar couplings (Fig. 14) for these three residues. Tryptophan contains a bulky aromatic system, which cannot rotate freely in the GB1 protein.
We have tried to apply the same procedure for the analysis of LC8 protein trying to find correlations between NMR measurements and quantum mechanical calculations of rotational barriers. Unfortunately, in the case of LC8 protein, the calculations are complicated by the fact that several polymorphic structures of LC8 are known. We have chosen the 3DVT structure for the calculations, but the calculated rotational barriers were not fully consistent with the experiment, which indicates that the 3DVT structure was not a good representative of the experimental structure.
A knowledge of the 3D structure of the studied molecule is crucial for the understanding of its mobility. If, however, the structural data are missing, the calculations of rotational barriers might be possibly used to provide structural constraints or even to differentiate between several structural models.
Conclusions
In conclusion, under fast MAS conditions, the measurements of heteronuclear (15N–1H and 13C–1H) dipolar couplings in proteins can be obtained in a site-resolved way in a 3D experiment containing the CPVC dipolar dimension and two isotropic chemical shift dimensions. We have demonstrated that, for aromatic sidechains, 13C–1H dipolar couplings can be accurately recorded and that they provide information on dynamic processes occurring on the timescales of dipolar interaction, as we have illustrated for two proteins, GB1 and LC8. Remarkably, by the analysis of dipolar lineshapes, specific motional modes can be inferred. Furthermore, in YAF, GB1, and LC8, the observed dynamic behavior could be readily accounted for by the analysis of tertiary contacts formed between the aromatic sidechains and other amino acids, and these observations are generally consistent with quantum mechanical calculations of the rotational energy barriers. Broadly, we expect that the methods introduced in this work will be useful for probing nano- to microsecond time scale motions in a variety of biological systems.Acknowledgements
The authors are grateful to the Polish National Center of Sciences (NCN) for financial support, Grant No. 2014/13/B/ST4/0350 and IR-RMN THC FR3050 for conducting the research. T. P. acknowledges the support of the National Institutes of Health (NIH Grants R01GM085306 and P50GM082251) as well as 1P30GM110758-01 for the support of core research infrastructure at the University of Delaware and the Erasmus Mundus Scholarship as a Visiting Professor at the University of Lille, M. D. acknowledges the support of the Czech Science Foundation (Grant No. 15-11223S).Notes and references
- M. Renault, A. Cukkemane and M. Baldus, Angew. Chem., Int. Ed., 2010, 49, 8346–8357 CrossRef CAS PubMed.
- M. Weingarth and M. Baldus, Acc. Chem. Res., 2013, 46, 2037–2046 CrossRef CAS PubMed.
- M. Tang, G. Comellas and C. M. Rienstra, Acc. Chem. Res., 2013, 46, 2080–2088 CrossRef CAS PubMed.
- S. Yan, C. L. Suiter, G. Hou, H. L. Zhang and T. Polenova, Acc. Chem. Res., 2013, 46, 2047–2058 CrossRef CAS PubMed.
- A. Loquet, B. Habenstein and A. Lange, Acc. Chem. Res., 2013, 46, 2070–2079 CrossRef CAS PubMed.
- A. Goldbourt, Curr. Opin. Biotechnol., 2013, 24, 705–715 CrossRef CAS PubMed.
- S. Asami and B. Reif, Acc. Chem. Res., 2013, 46, 2089–2097 CrossRef CAS PubMed.
- A. D. Torchia, J. Magn. Reson., 2011, 212, 1–10 CrossRef PubMed.
- T. Kobayashi, K. Mao, P. Paluch, A. Nowak-Krol, J. Sniechowska, Y. Nishiyama, D. T. Gryko, M. J. Potrzebowski and M. Pruski, Angew. Chem., Int. Ed., 2013, 52, 14108–14111 CrossRef CAS PubMed.
- V. Agarwal, S. Penzel, K. Szekely, R. Cadalbert, E. Testori, A. Oss, J. Past, A. Samoson, M. Ernst, A. Bockmann and B. H. Meier, Angew. Chem., Int. Ed., 2014, 53, 12253–12256 CrossRef CAS PubMed.
- Q. Wang, X. Lu, O. Lafon, J. Trebosc, F. Deng, B. Hu, Q. Chen and J. P. Amoureux, Phys. Chem. Chem. Phys., 2011, 13, 5967–5973 RSC.
- G. Hou, X. Lu, A. J. Vega and T. Polenova, Chem. Phys., 2014, 141, 104202 Search PubMed.
- A. Gansmüller, J.-P. Simorre and S. Hediger, J. Magn. Reson., 2013, 234, 154–164 CrossRef PubMed.
- M. F. Cobo, D. Reichert, K. Saalwächter and E. R. deAzevedo, J. Magn. Reson., 2014, 248, 115–125 CrossRef CAS PubMed.
- P. Schanda, B. H. Meier and M. Ernst, J. Magn. Reson., 2011, 210, 246–259 CrossRef CAS PubMed.
- M. H. Levitt, Symmetry-Based Pulse Sequence in Magic-Angle Spinning Solid-State NMR, Encyclopedia of Magnetic Resonance, Springer, 2007 Search PubMed.
- G. Hou, I. J. L. Byeon, J. Ahn, A. M. Gronenborn and T. Polenova, J. Chem. Phys., 2012, 137, 134201 CrossRef PubMed.
- G. Hou, X. Lu, A. J. Vega and T. Polenova, J. Chem. Phys., 2014, 141, 104202 CrossRef PubMed.
- P. Paluch, T. Pawlak, J. P. Amoureux and M. J. Potrzebowski, J. Magn. Reson., 2013, 233, 56–63 CrossRef CAS PubMed.
- P. Paluch, J. Trebosc, Y. Nishiyama, M. J. Potrzebowski, M. Malon and J. P. Amoureux, J. Magn. Reson., 2015, 252, 67–77 CrossRef CAS PubMed.
- H. P. Park, C. Yang, S. J. Opella and J. M. Mueller, J. Magn. Reson., 2013, 237, 164–168 CrossRef PubMed.
- R. Zhang, J. Damron, T. Vosegaard and A. Ramamoorthy, J. Magn. Reson., 2015, 250, 37–44 CrossRef CAS PubMed.
- M. M. Slabicki, M. J. Potrzebowski, G. Bujacz, S. Olejniczak and J. Olczak, J. Phys. Chem. B, 2004, 108, 4535–4545 CrossRef CAS.
- S. Sun, A. H. Butterworth, S. Paramasivam, S. Yan, C. M. Lightcap, J. C. Williams and T. Polenova, Can. J. Chem., 2011, 89, 909–918 CrossRef CAS PubMed.
- R. S. Thakur, N. D. Kurur and P. K. Madhu, Chem. Phys. Lett., 2006, 426, 459–463 CrossRef CAS PubMed.
- R. S. Thakur, N. D. Kurur and P. K. Madhu, J. Magn. Reson., 2008, 193, 77–88 CrossRef CAS PubMed.
- M. Shen, B. Hu, O. Lafon, J. Trébosc, Q. Chen and J. P. Amoureux, J. Magn. Reson., 2012, 223, 107–119 CrossRef CAS PubMed.
- E. O. Stejskal, J. Schaefer and J. S. Waugh, J. Magn. Reson., 1977, 28, 105–112 CAS.
- H. Meier, Chem. Phys. Lett., 1992, 188, 201–207 CrossRef.
- X. Wu and K. W. Zilm, J. Magn. Reson., Ser. A, 1993, 104, 154–165 CrossRef CAS.
- F. Marica and R. F. Snider, Solid State Nucl. Magn. Reson., 2003, 23, 28–49 CrossRef CAS.
- P. Schanda, M. Huber, J. Boisbouvier, B. H. Meier and M. Ernst, Angew. Chem., Int. Ed., 2011, 50, 11005–11009 CrossRef CAS PubMed.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef PubMed.
- E. R. McNellis, J. Meyer and K. Reuter, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 205414 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- http://www.rcsb.org/Protein Data Bank .
- H. L. F. Schmidt, L. J. Sperling, Y. G. Gao, B. J. Wylie, J. M. Boettcher, S. R. Wilson and C. A. Rienstra, J. Phys. Chem. B, 2007, 111, 14362–14369 CrossRef PubMed.
- T. Pawlak, K. Trzeciak-Karlikowska, J. Czernek, W. Ciesielski and M. J. Potrzebowski, J. Phys. Chem. B, 2012, 116, 1974–1983 CrossRef CAS PubMed.
- M. Kamihira, A. Naito, S. Tuzi and H. J. Saito, J. Phys. Chem. A, 1999, 103, 3356–3363 CrossRef CAS.
- A. Naito, T. Iizuka, S. Tuzi, W. S. Price, K. Hayamizu and H. Saito, J. Mol. Struct., 1995, 355, 55–60 CrossRef CAS.
- A. Rapp, I. Schnell, D. Sebastiani, S. P. Brown, V. Percec and H. W. Spiess, J. Am. Chem. Soc., 2003, 125, 13284–13297 CrossRef CAS PubMed.
- H. N. B. Moseleyl, L. J. Sperling and C. M. Rienstra, J. Biomol. NMR, 2010, 48, 123–128 CrossRef PubMed.
- H. Zhou, G. Shah, M. Cormos, C. Mullen, D. Sando and C. M. Rienstra, J. Am. Chem. Soc., 2007, 129, 11791 CrossRef PubMed.
- L. J. Chen, M. Kaiser, J. Lai, T. Polenova, J. Yang, C. M. Rienstra and L. Mueller, Magn. Reson. Chem., 2007, 45, 84–92 CrossRef PubMed.
- W. T. Franks, D. H. Zhou, B. J. Wylie, B. G. Money, D. T. Graesser, H. L. Frericks, G. Sahota and C. M. Rienstra, J. Am. Chem. Soc., 2005, 127, 12291–12305 CrossRef CAS PubMed.
- G. Hou, I. L. Byeon, J. Ahn, A. M. Gronenborn and T. Polenova, J. Am. Chem. Soc., 2011, 133, 18646–18655 CrossRef CAS PubMed.
- G. Hou, S. Paramasivam, I. L. Byeon, A. M. Gronenborn and T. Polenova, Phys. Chem. Chem. Phys., 2010, 12, 14873–14883 RSC.
- S. J. Sun, A. H. Butterworth, S. Paramasivam, S. Yan, C. M. Lightcap, J. C. Williams and T. Polenova, Can. J. Chem., 2011, 89, 909–918 CrossRef CAS PubMed.
- C. Guo, G. Hou, X. Lu, B. O'Hare, J. Struppe and T. Polenova, J. Biomol. NMR, 2014, 60, 219–229 CrossRef CAS PubMed.
- M. Karplus and J. A. McCammon, Nat. Struct. Biol., 2002, 9, 646–652 CrossRef CAS PubMed.
- J. L. Klepeis, K. Lindorff-Larsen, R. O. Dror and D. E. Shaw, Curr. Opin. Struct. Biol., 2009, 19, 120–127 CrossRef CAS PubMed.
- L. A. Abriata and M. Dal Peraro, Sci. Rep., 2015, 5, 10549, DOI:10.1038/srep10549.
- M. Dračínský, P. Jansa, K. Ahonen and M. Buděšínský, Eur. J. Org. Chem., 2011, 1544–1551 CrossRef PubMed.
- A. Hunter and J. K. M. Anders, J. Am. Chem. Soc., 1990, 112, 5525–5534 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cp04475h |
| This journal is © the Owner Societies 2015 |