 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Combined near-infrared excited SEHRS and SERS spectra of pH sensors using silver nanostructures†
Marina
Gühlke‡
a,
Zsuzsanna
Heiner‡
ab and
Janina
Kneipp
*ab
aDepartment of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, 12489 Berlin, Germany. E-mail: janina.kneipp@chemie.hu-berlin.de
bSchool of Analytical Sciences Adlershof SALSA, Humboldt-Universität zu Berlin, Zum Großen Windkanal 6, 12489 Berlin, Germany
First published on 26th August 2015
Abstract
Surface-enhanced hyper-Raman scattering (SEHRS) and surface-enhanced Raman scattering (SERS) of para-mercaptobenzoic acid (pMBA) were studied with an excitation wavelength of 1064 nm, using different silver nanostructures as substrates for both SEHRS and SERS. The spectra acquired for different pH values between pH 2 and pH 12 were compared with SERS data obtained from the identical samples at 532 nm excitation. Comparison of the ratios of the enhancement factors from SEHRS and SERS experiments with those from calculations using plasmonic absorbance spectra suggests that the difference between total surface-enhancement factors of SEHRS and SERS for pMBA is mainly explained by a difference between the electromagnetic contributions for linear and non-linear SERS. SERS and SEHRS spectra obtained at near-infrared (NIR) excitation indicate an overall reduction of enhancement by a factor of 2–3 at very low and very high pH, compared to neutral pH. Our data provide evidence that different molecular vibrations and/or different adsorption species are probed in SERS and SEHRS, and that SEHRS is very sensitive to slight changes in the pMBA–nanostructure interactions. We conclude that the combination of SEHRS and SERS using NIR excitation is more powerful for micro-environmental pH sensing than one-photon spectra excited in the visible range alone.
Introduction
Vibrational spectroscopies, such as Raman and infrared spectroscopies, can be employed to characterize the structure, composition, and interaction of molecules and materials in a variety of analytical applications. Especially the combination of the one-photon excited linear Raman scattering and the two-photon excited non-linear hyper-Raman scattering, which occurs near the second harmonic of the excitation wavelength, can give a lot of structural information, because hyper-Raman scattering can offer information complementary to that from Raman spectroscopy and has some advantages over Raman and IR spectroscopies due to its different selection rules. The Raman-active vibrational modes of a centrosymmetric molecule are hyper-Raman-forbidden, and those inactive in both IR and Raman can be active in hyper-Raman scattering.1 In the presence of metal nanostructures, both one- and two-photon excited Raman scattering can profit from surface-enhancement. Surface-enhanced Raman scattering (SERS) allows us to capture data about the molecular structure and composition with high signal intensities in the strongly confined local electromagnetic fields of plasmonic nanostructures.2,3 In surface-enhanced hyper-Raman scattering, the inherently low two-photon cross-sections of the nonlinear hyper-Raman scattering can be overcome and increase to an order of 10−46–10−45 cm4 s.4In the excitation range between 1000 nm and 1300 nm, mostly Fourier-transform (FT) techniques have been used in Raman spectroscopy; only in the past few years, technological advances allowed a more widespread use of the dispersive technique because of the revolution of non-silicon-based detectors.5,6 Thus, a direct comparison of Raman and hyper-Raman scattering, both excited at near infrared (NIR) wavelengths, but occuring at NIR and visible wavelengths, respectively, is possible. Nevertheless, in dispersive Raman spectroscopy at near infrared (NIR) wavelengths, the low Raman scattering intensities and detector sensitivities require long acquisition times. This can be improved by SERS, which was observed for NIR excitation first in FT-Raman on electrodes7–9 as well as on silver nanoparticles.10,11 Compared with its SERS spectrum, the SEHRS spectrum of a centrosymmetric molecule can contain new vibrational bands and display significant relative intensity differences because of the surface effect.12 In contrast, the two-photon excited spectrum of a non-centrosymmetric molecule largely resembles its SERS spectrum.4,13,14 Since the two processes give different insights into molecular symmetry, the combination of one- and two-photon NIR-excited SERS is a comprehensive way to investigate different molecules and molecule–nanostructure interactions.
An example of a molecule displaying qualitatively different SERS and SEHRS spectra is para-mercaptobenzoic acid (pMBA)15 which, similar to other organothiols,16 can strongly attach to silver nanostructures via its thiol group.17,18 Concentration-dependent changes of the orientation of the molecules on the silver surface have been monitored using the SERS spectra.19 Furthermore, in these spectra, the protonation and deprotonation of the carboxylate group in different pH environments can be observed,19 therefore the pMBA molecule can be used as a pH nanosensor, e.g., in the endosomal system of live cells.15,18,20pMBA-based SERS pH sensors typically enable monitoring of a pH range of approximately pH 3.5 to pH 9 on silver and gold nanostructures.15,21,22 A first study employing two-photon excitation with pMBA on citrate-stabilized silver nanoparticles has suggested to use it for other pH ranges than excitation in the one-photon regime, specifically, the pH range of a SEHRS sensor could be extended to pH 2 in the acidic range.15
Here we discuss SEHRS spectra of pMBA acquired between pH 2 and 12 under non-resonant conditions in one combined microspectroscopic setup, together with the visible and near-infrared excited SERS spectra. Specifically, we report both SERS and SEHRS data obtained at an excitation wavelength of 1064 nm, and we show that this combination is more powerful for the determination of micro-environmental pH (e.g., in biomaterials) and for the characterization of the sensor than one-photon spectra excited in the visible range at 532 nm alone. To better understand possible origins of the high pH sensitivity of NIR-excited SEHRS and SERS we have used several kinds of silver nanostructures.
Materials and methods
Chemicals
Silver nitrate (99.9999%), hydroxylamine hydrochloride (99%), sodium hydroxide (p.a.) and para-mercaptobenzoic acid (pMBA) (99%) were purchased from Sigma-Aldrich. Trisodium citrate dihydrate (99%) was purchased from Th. Geyer, and sodium borohydride and hydrochloric acid were purchased from J. T. Baker. Phosphate buffers of different pH values with a phosphate concentration of 0.1 M were prepared according to Sörensen's protocol23 with potassium dihydrogen phosphate (p.a., Merck) and disodium hydrogen phosphate (99%, Sigma-Aldrich).Synthesis of the pH nanosensors/sample preparation
Silver nanoparticles (AgNPs) were produced by chemical reduction of silver nitrate according to four different protocols. For citrate reduced AgNPs,24 5 mL of a 0.04 M sodium citrate solution were added to a boiling solution of 45 mg of silver nitrate in 245 mL of water. The mixture was kept boiling for 1 hour. For hydroxylamine reduced AgNPs,25 17 mg of silver nitrate were dissolved in 10 mL of water and added to 90 mL of an aqueous solution containing 12 mg of sodium hydroxide and 11 mg of hydroxylamine hydrochloride. The mixture was stirred for 1 hour. For the production of NaBH4 reduced, citrate stabilized AgNPs, two different procedures were used: (I) in the first procedure,26 a solution of 4.3 mg of silver nitrate in 250 mL of water was cooled to 4 °C and 0.9 mmol sodium citrate were added. Afterwards 1.25 × 10−3 mmol NaBH4 were added from a freshly prepared concentrated aqueous solution. The resulting particles are further referred to as Ag (NaBH4/citrate I). (II) In the second procedure,27 0.65 mL of a 0.03 mM sodium citrate solution were added at 0 °C to 6.5 mL of a 1 mM AgNO3 solution. Afterwards, 0.35 mL of a 0.1 M NaBH4 solution were added and the mixture was stirred at 0 °C for 30 minutes. The resulting particles are further referred to as Ag (NaBH4/citrate II).To form the nanosensors, silver nanoaggregates were mixed with pMBA to a final pMBA concentration of 9 × 10−7 M. pH was adjusted by ten times diluting the nanosensor samples with 0.1 M phosphate buffer solutions. Extremely high and low pH values were achieved by the addition of sodium hydroxide and hydrochloric acid, respectively.
Characterization of the nanosensors
Absorbance spectra were recorded on a Jasco UV/visible/NIR spectrophotometer.Transmission electron microscopy (TEM) images were obtained on holey carbon Cu-grids using a TECNAI G220 S-TWIN and a Jeol JEM 2200-FS electron microscope operating at 200 kV.
Raman experiments
The surface-enhanced Raman and hyper-Raman spectra were obtained using an imaging spectrometer by microprobe sampling (10× objective). For detection, a 1200 lines per mm grating blazed at 550 nm with a liquid nitrogen cooled CCD detector and a 600 lines per mm grating blazed at 1000 nm with a liquid nitrogen cooled InGaAs detector were used in the visible and NIR spectral range, respectively. The liquid samples were placed in microcontainers, and the one- and two-photon scattering was collected in confocal and epi-illumination. One-photon excited SERS spectra were typically accumulated for 1 s in the visible and for 10 s in the NIR range with laser powers of 10 mW from a 532 nm CW laser and 500 mW from a 1064 nm mode-locked laser. Hyper-Raman excitation with 300 mW at 1064 nm was provided by a mode-locked laser producing 7 ps pulses at a 76 MHz repetition rate. SEHRS spectra were accumulated for 2–15 s. Spectral resolution was 3–6 cm−1 in the full spectral range.Estimation of cross-sections and enhancement from Raman experiments
The scattering power in SERS and SEHRS, PSERS and PSEHRS, measured in counts per second, was estimated at 1075 cm−1 and 1069 cm−1 (ring breathing mode) by SERS and SEHRS, respectively, according to| PSERS = N0σSERSnL | (1) |
| PSEHRS = N0σSEHRSnL2 | (2) |
Estimation of enhancement from absorbance spectra
The absorbance spectra can be used to determine empirically the electromagnetic SERS and SEHRS enhancement factors, GSERS and GSEHRS, that can be obtained with the different nanoparticles according to eqn (3)30 and eqn (4),14 respectively | (3) |
 | (4) |
![[small epsilon, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0de.gif) (ωl) = ε′(ωl) + iε′′(ωl),
(ωl) = ε′(ωl) + iε′′(ωl), ![[small epsilon, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0de.gif) (ωS) = ε′(ωS) + iε′′(ωS), and
(ωS) = ε′(ωS) + iε′′(ωS), and ![[small epsilon, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0de.gif) (ωH) = ε′(ωH) + iε′′(ωH), respectively.
(ωH) = ε′(ωH) + iε′′(ωH), respectively.
Results and discussion
Absorbance spectra and enhancement factors
Different types of silver nanostructures were synthesized by reduction with citrate, hydroxylamine, and sodium borohydride (following two different protocols), respectively. Transmission electron micrographs (TEM) of the different kinds of silver nanoparticles are shown in Fig. 1a–d. While citrate reduction gives relatively large particles with a significant amount of rod-like shapes (Fig. 1a), hydroxylamine reduction yields mostly spherical particles in a similar size (Fig. 1b). Reduction with borohydride, combined with citrate stabilization, either results in relatively small particles with an average diameter of 14 nm and a small fraction of triangular shaped nanoparticles (synthesis procedure I, Fig. 1c) or in large agglomerates (synthesis procedure II, Fig. 1d). Corresponding to the different size and shape of the nanoparticles, the nanosensors, consisting of these nanoparticles and pMBA, show variations in the position of the maximum absorbance and the shape of the spectra at neutral pH (Fig. 1e). The spectra of the sensors containing Ag (citrate) or Ag (hydroxylamine) are very reproducible for the pH range from pH 2 to 12 (ESI,† Fig. S1), indicating the stability of the nanoparticle solutions and their applicability for experiments with varying pH values. As Fig. 1f shows, the absorbance at 1064 nm of each nanoparticle solution is stable for different experiments conducted at acidic and neutral pH, while at basic pH slight variations in the extended plasmon band can be observed. We use this wavelength for the excitation of normal and hyper-Raman scattering and observe high enhancement in the SEHRS and SERS spectra despite the large gap between the excitation wavelength and the plasmon resonance maximum. This is in accord with previous theoretical and experimental findings, which describe high enhancement when frequency is changed from UV to near-IR wavelengths.32–34The ratio of the electromagnetic enhancement of one-photon excited Raman scattering and hyper-Raman scattering can be estimated for a given absorbance spectrum of the sample and for a given excitation wavelength. Table 1 displays this ratio for the signal of the ring breathing vibration at around 1070 cm−1 at an excitation wavelength of 1064 nm at pH 7 (cf. spectra in Fig. 1e) resulting from eqn (3) and (4). In the case of Ag (hydroxylamine) nanoaggregates, we find a ratio of ∼10−5, while the ratio is ∼10−4 for Ag (citrate).
| Ag nanoparticles at pH 7 | Hydroxylamine | Citrate | NaBH4/citrate I |
|---|---|---|---|
| a No SERS signal was obtained. | |||
| σ SERS/σSEHRS [photons cm−2 s−1] | 2 × 1027 | 3 × 1027 | n.d.a |
| G SERS/GSEHRS from Raman experiments | 9 × 10−4 | 8 × 10−4 | n.d.a |
| G SERS/GSEHRS from EM field theory | 3 × 10−5 | 9 × 10−4 | 6 × 10−8 |
| Focal volume [μm3] | |||
| In SERS | 290 | 290 | 290 |
| In SEHRS | 37 | 37 | 37 |
| Average particle diameter [nm] | 42 ± 15 | 131 ± 29 | 14 ± 6 |
| Number of molecules in the focal volume | |||
| In SERS | 2 × 105 | 2 × 105 | 2 × 105 |
| In SEHRS | 2 × 104 | 2 × 104 | 2 × 104 |
| Number of particles in the focal volume | |||
| In SERS | 11 | 0.24 | 19 |
| In SEHRS | 1 | 0.03 | 2 |
Using SEHRS and SERS spectra obtained at pH 7, we have estimated the enhancement by the different silver nanostructures directly from Raman experiments. Example SEHRS and SERS spectra excited at 1064 nm are shown in Fig. 2 and 5, respectively. The different ratios of the cross-sections are also listed in Table 1. The enhancement factor ratios obtained from the Raman spectra agree with the empirical values from the absorbance spectra using eqn (3) and (4). This comparison shows that, in the case of pMBA, the difference between the total surface-enhancement factors of SEHRS and SERS can be explained by the difference between the corresponding electromagnetic contributions to the enhancement.
pH-dependent SEHRS and SERS spectra
SEHRS spectra of pMBA were obtained at varying pH with silver nanostructures prepared according to four different protocols. The SEHRS spectra for pH 2, pH 7, and pH 12 are displayed as examples in Fig. 2. The Ag (NaBH4/citrate II) nanoparticles cause the formation of very large aggregates (see TEM in Fig. 1d), and a high background signal in all SEHRS experiments (Fig. 2d). In spite of their high stability (see also absorbance data in Fig. 1f), they are therefore not suitable for the construction of pH nanosensors. The spectra in Fig. 2a show the characteristic SEHRS signature of pMBA that was reported for citrate-reduced silver nanoaggregates previously.15 The spectra of pMBA obtained with Ag (hydroxylamine) (Fig. 2b) and Ag (NaBH4/citrate I) (Fig. 2c) are very similar for the corresponding pH values. They display differences in some relative band intensities compared to the spectra in Fig. 2a, due to different interactions of the pMBA molecules with the nanostructures. The high similarity of the spectra in Fig. 2b and c appears in spite of the high dissimilarity in surface coverage: while pMBA is present in excess on Ag (NaBH4/citrate I), leading to full surface coverage considering a space requirement given in ref. 28, the Ag (hydroxylamine) particles are covered to a much lesser extent (∼50%).With increasing pH, in all spectra, the relative band intensities of vibrations associated with the carboxyl group (marked in green in Fig. 2) change relative to those of the aromatic ring (marked in blue in Fig. 2). Upon deprotonation, the intensity of the COO− band at 1365 cm−1 increases, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1685 cm−1 decreases. This is in accord with the pH dependence of the one-photon SERS spectra excited at 532 nm (ESI,† Fig. S2).
O stretching vibration at 1685 cm−1 decreases. This is in accord with the pH dependence of the one-photon SERS spectra excited at 532 nm (ESI,† Fig. S2).
Comparing the SEHRS spectra in Fig. 2 with the one-photon excited SERS spectra (ESI,† Fig. S2), we find in the SEHRS spectra smaller Raman shifts by 5–15 cm−1 for several bands (Fig. 2 and ESI,† Table S1). This is not specific for one nanoparticle type. These bands, which are seemingly shifted, can also indicate probing of different vibrations in SERS and SEHRS due to the different selection rules,12 and can be an indication that different adsorption species are probed in SERS and SEHRS. It is known that SEHRS is more sensitive to surface potential13 and to local surface environmental changes12 than SERS.
For high pH values, new band components, such as asymmetric broadening and new shoulders, appear, which can also be an indication that different adsorption species are probed in SERS and SEHRS. Fig. 3a displays this for the example of the ring stretching vibration at 1585 cm−1 in the SEHRS spectra, while the band in the SERS data is only shifted and shows no second component (Fig. 3b and c). The appearance of a low-frequency shoulder in the SEHRS spectrum can be assigned to the non-totally symmetric ring stretching vibration, whereas the high-frequency component corresponds to the totally symmetric ring stretching vibration. The former was reported in SERS data as well, upon increasing charge transfer between the molecule and the silver surface,35 or when intermolecular interaction between the phenyl ring and a carboxylate group takes place.36 This indicates that at high pH, a larger fraction of the molecules must be adsorbed on the surface in a flat orientation and can be specifically probed by SEHRS (Fig. 3a). Apart from a small shift, this is not visible from the ring stretching band in the SERS spectra (Fig. 3b and c). In contrast, other bands in the SERS spectra support the same pH-dependent change in orientation found from the SEHRS data: for example, the low-frequency shoulder of the carboxylate stretching vibration at 1368 cm−1 (ESI,† Fig. S2) points to surface-bound carboxylate.37 The flat orientation is also evidenced by a higher intensity of bands from out-of-plane vibrations of the phenyl ring at 684 cm−1 and 710 cm−1 in both the SEHRS and SERS spectra (ESI,† Fig. S3) at neutral and basic pH.19,35,38 This is more pronounced in the SEHRS spectra, where both bands are clearly visible at neutral pH, and form one broad band with reduced intensity at acidic pH (ESI,† Table S1 and Fig. S3a). In the SERS spectra, only the broad band at 697 cm−1 and the band at 710 cm−1 are observed at acidic and neutral pH, respectively, and the C–H-deformation at 684 cm−1 does not appear (ESI,† Fig. S3b). These differences can be explained by the different selection rules that govern SEHRS, leading to preferred probing of those vibrations that are sensitive to pH-dependent surface interaction of the pMBA molecules. Similar effects were observed for other molecules.12,13
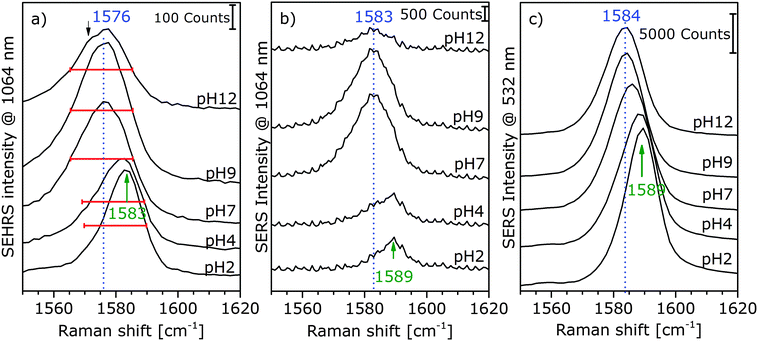 | ||
| Fig. 3 Extracts of SEHRS (a) and SERS spectra of pMBA with Ag (citrate) nanoparticles at an excitation wavelength of 1064 nm (b) and 532 nm (c). Experimental conditions as described in Fig. 2, 5, and Fig. S2 (ESI†), respectively. | ||
As proposed by us and others, pH sensing using pMBA SERS/SEHRS spectra relies on changes in relative intensities15,20,21 that are caused by both protonation and deprotonation of the molecule as well as by the resulting change in orientation at the nanoparticle surface.19 In Fig. 4a, the band ratio of the bands at 1365 cm−1 (COO− stretching) and at 1069 cm−1 (ring breathing) from the SEHRS spectra is displayed as a function of pH (red triangles in Fig. 4a). Using this band ratio, acidic pH values can be clearly distinguished. Even though the sensors are stable at high pH (Fig. 1b and ESI,† Fig. S1), a nearly constant intensity of the carboxyl band at 1365 cm−1 in the pMBA spectrum for values above pH 8 prevents the discrimination of very similar, basic pH values. This is due to the uniform complete deprotonation of the carboxyl group above pH 8 (pKa = 5 for the carboxyl group and pKa = 5.8 for the thiol group).39 Therefore, the intensity ratio of pH-sensitive carboxyl and pH-insensitive aromatic bands in the SEHRS spectra is well-suited for the differentiation of acidic and neutral pH-values. From the comparison of the intensity ratios at different pH values for the different kinds of nanoparticles (Fig. 4b) we see that the sensitivity of a sensor made with citrate-stabilized silver nanostructures is highest, specifically in the acidic pH range. In Fig. 4a, we also compare the intensity ratios in the 1064 nm excited SEHRS spectra with the same intensity ratios in SERS spectra excited at 532 nm (black squares, for spectra please refer to ESI,† Fig. S2). The similar trend for the intensity ratio in the two types of spectra shows that, besides providing additional information on the interaction between the molecules and the metal surface, SEHRS spectra also contain the pH dependent spectral information that is obtained from the SERS spectra.
 | ||
| Fig. 4 (a) Intensity ratios in the SERS spectra excited at 532 nm and in the SEHRS spectra excited at 1064 nm of pMBA with citrate reduced/stabilized NPs plotted as a function of pH for the bands at 1365 and 1069 cm−1 to demonstrate the operating range of SERS and SEHRS based pH-probes. (b) SEHRS intensity ratios in the spectra of pMBA with citrate (black), hydroxylamine (red) and NaBH4 (green) reduced AgNPs as a function of pH for the bands at 1365 and 1178 cm−1. One-photon excitation: 532 nm (CW), intensity: 3 × 105 W cm−2, acquisition time: 1 s, and pMBA concentration: 9 × 10−7 M. Two-photon excitation: 1064 nm (7 ps/76 MHz), photon flux density: 2 × 1025 photons cm−2 s−1, acquisition time: 2 s, and pMBA concentration: 9 × 10−7 M. Positions of the bands are given according to the SEHRS spectra, for a comparison of band positions in SEHRS and SERS spectra see ESI,† Table S1. Intensity ratios are averaged over 30 spectra and error bars represent the corresponding standard deviations. | ||
Considering the optimum pH range of the sensor in the acidic and neutral range, an application to biological systems, where the advantages of 2-photon excitation regarding material damage, spatial resolution, and penetration depth are obvious, using excitation at an infrared wavelength also for one-photon excitation is desirable. In Fig. 5a, we show SERS spectra that were excited with 1064 nm by the same laser as the SEHRS spectra and can be detected quasi-simultaneously in our microspectroscopic setup. Here, we utilize the intensity ratio of the pH-sensitive band at 363 cm−1 and of the pH-insensitive band at 523 cm−1 of a phenyl deformation vibration (see the band assignment in ESI,† Table S1), to discriminate pH values in the range between 2 and 7 (Fig. 5b). The band at 363 cm−1 can be assigned to deprotonated pMBA,28 particularly to a phenyl deformation combined with the C–S-stretching vibration38,40 and increases in intensity with pH becoming less acidic (Fig. 5a). Since its intensity is much stronger than that of the band at 710 cm−1 of the out-of-plane phenyl γ(C C C), that is used in the SEHRS spectra to probe the molecular orientation, it can serve as a very sensitive indicator for pH-induced changes in molecular orientation.
pH-dependent enhancement of SEHRS and SERS
All nanoparticle types show a reduction of SEHRS enhancement by a factor of 2–3 at very low and very high pH, compared to neutral pH (Fig. 2a–d). The effect is strongest for the Ag (hydroxylamine) nanoparticles (Fig. 2b) and for Ag (NaBH4/citrate I) (Fig. 2c). Also, one-photon SERS excited at 1064 nm (Fig. 5a) shows similar behavior, i.e. an increase of the overall signal when the pH changes from acidic to neutral, and a decrease again at high pH. This effect is not observed in 532 nm excited SERS spectra with the same samples (ESI,† Fig. S2). Especially for the acidic pH values, the change in overall enhancement supports an influence of the surface chemistry, such as an altered interaction of the molecule with the metal nanoparticle surface, as was proposed previously,19 or with the stabilizing species.41 In particular, the stabilizing citrate and hydroxylamine/hydroxide contain functional groups that can be protonated or deprotonated, depending on the surrounding pH.Due to the resulting changes in the nanoparticle surface, charge aggregation of the nanosensors, rather than severe Ag-nanostructure modifications that were observed as a function of the concentration of some organic molecules,42 could take place: for example, the negative surface charge of the silver particles decreases with decreasing pH by protonation, leading to increased aggregation and minor changes in gap sizes in the nanoaggregates and therefore to a lower electromagnetic enhancement at very acidic pH.43,44 This can remain undetected in the absorbance spectra if occurring only for a small fraction of the nanoparticles,45 but it could greatly change electromagnetic enhancement for some of the nanosensors.46
Apart from changes to the stabilizing species, a protonation of the thiolate could weaken the Ag–S-bond and thus change the interaction between pMBA itself and the silver surface at acidic pH. On the opposite side, at basic pH, where both, the stabilizing molecules and pMBA, are deprotonated, the repulsion between the negative charges of the analyte molecule and the nanoparticle surface is high,43 which alters the interaction between the metal surface and pMBA and thus leads to decreased enhancement. At neutral and slightly basic pH the two effects are balanced, and the interaction of the molecule with the metal surface leads to the highest enhancement.
Conclusions
In conclusion, the combination of near-infrared excited SEHRS and SERS on the same sample, in one microspectroscopic setup along with the option to measure SERS using the second harmonic of the excitation laser, offers new possibilities for comparing one- and two-photon-excited SERS. This can provide deeper insight into the enhancement mechanism in linear and non-linear SERS and for the examination of interactions of molecules with different metal nanostructures. Here, we have investigated SERS and SEHRS of pMBA on silver nanostructures in a varying pH environment.Our experiments identify advantages of SEHRS over SERS regarding improved vibrational spectroscopic selectivity and an extended pH-detection range as can be seen from the SEHRS spectra of pMBA at varying pH. In addition, as a two-photon excited spectroscopy, SEHRS benefits from a decreased probed volume.
Our studies suggest new possibilities regarding sensing applications by exploiting combined SEHRS/SERS measurements using NIR excitation. As we have shown, a combined SEHRS/SERS pH sensor which uses pMBA is most sensitive in the acidic and neutral pH ranges. This makes it especially useful for the examination of biological objects, which generally profits from near-infrared excitation. For future applications, combining nonlinear and linear NIR excitation in SERS, together with the tunable optical properties of plasmonic nanoparticles, will open up new possibilities for microscopic bio-sensing.
Acknowledgements
We thank Dr Harald Kneipp for valuable discussions and support in setting up experiments. We thank Tina Büchner for providing Ag (NaBH4/citrate I) nanoparticles and Dr Virginia Merk and Sebastian Fredrich for providing Ag (NaBH4/citrate II) nanoparticles. We also thank Sören Selve and Dr Guillermo Orts-Gil for help with TEM. Funding by ERC Starting Grant no. 259432 (MULTIBIOPHOT) to all authors and by DFG (GSC 1013 SALSA) to Z.H. is gratefully acknowledged.References
- S. J. Cyvin, J. E. Rauch and J. C. Decius, J. Chem. Phys., 1965, 43, 4083–4095 CrossRef CAS.
- C. D'Andrea, B. Fazio, P. G. Gucciardi, M. C. Giordano, C. Martella, D. Chiappe, A. Toma, F. B. de Mongeot, F. Tantussi, P. Vasanthakumar, F. Fuso and M. Allegrini, J. Phys. Chem. C, 2014, 118, 8571–8580 CrossRef.
- Y. S. Yamamoto, Y. Ozaki and T. Itoh, J. Photochem. Photobiol., C, 2014, 21, 81–104 CrossRef CAS.
- J. Kneipp, H. Kneipp and K. Kneipp, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 17149–17153 CrossRef CAS PubMed.
- A. Rogalski, Opto-Electron. Rev., 2012, 20, 279–308 Search PubMed.
- A. Rogalski, Prog. Quantum Electron., 2012, 36, 342–473 CrossRef.
- S. M. Angel, L. F. Katz, D. D. Archibald, L. T. Lin and D. E. Honigs, Appl. Spectrosc., 1988, 42, 1327–1331 CrossRef CAS.
- D. B. Chase and B. A. Parkinson, Appl. Spectrosc., 1988, 42, 1186–1187 CrossRef CAS.
- A. Crookell, M. Fleischmann, M. Hanniet and P. J. Hendra, Chem. Phys. Lett., 1988, 149, 123–127 CrossRef CAS.
- R. Prucek, A. Panacek, A. Fargasova, V. Ranc, V. Masek, L. Kvitek and R. Zboril, CrystEngComm, 2011, 13, 2242–2248 RSC.
- M. W. Meyer and E. A. Smith, Analyst, 2011, 136, 3542–3549 RSC.
- J. C. Hulteen, M. A. Young and R. P. van Duyne, Langmuir, 2006, 22, 10354–10364 CrossRef CAS PubMed.
- S. M. Nie, L. A. Lipscomb and N. T. Yu, Appl. Spectrosc. Rev., 1991, 26, 203–276 CrossRef.
- H. Kneipp, K. Kneipp and F. Seifert, Chem. Phys. Lett., 1993, 212, 374–378 CrossRef CAS.
- J. Kneipp, H. Kneipp, B. Wittig and K. Kneipp, Nano Lett., 2007, 7, 2819–2823 CrossRef CAS PubMed.
- J. Kubackova, I. Izquierdo-Lorenzo, D. Jancura, P. Miskovsky and S. Sanchez-Cortes, Phys. Chem. Chem. Phys., 2014, 16, 11461–11470 RSC.
- S. M. Ansar, G. S. Perera, P. Gomez, G. Salomon, E. S. Vasquez, I. W. Chu, S. L. Zou, C. U. Pittman, K. B. Walters and D. M. Zhang, J. Phys. Chem. C, 2013, 117, 27146–27154 CrossRef CAS.
- C. E. Talley, L. Jusinski, C. W. Hollars, S. M. Lane and T. Huser, Anal. Chem., 2004, 76, 7064–7068 CrossRef CAS PubMed.
- A. Michota and J. Bukowska, J. Raman Spectrosc., 2003, 34, 21–25 CrossRef CAS.
- J. Kneipp, H. Kneipp, B. Wittig and K. Kneipp, J. Phys. Chem. C, 2010, 114, 7421–7426 CrossRef CAS.
- S. W. Bishnoi, C. J. Rozell, C. S. Levin, M. K. Gheith, B. R. Johnson, D. H. Johnson and N. J. Halas, Nano Lett., 2006, 6, 1687–1692 CrossRef CAS PubMed.
- A. M. Schwartzberg, T. Y. Oshiro, J. Z. Zhang, T. Huser and C. E. Talley, Anal. Chem., 2006, 78, 4732–4736 CrossRef CAS PubMed.
- S. P. L. Sörensen, Biochem. Z., 1909, 21, 131–304 Search PubMed.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef CAS.
- N. Leopold and B. Lendl, J. Phys. Chem. B, 2003, 107, 5723–5727 CrossRef CAS.
- X. Y. Dong, X. H. Ji, J. Jing, M. Y. Li, J. Li and W. S. Yang, J. Phys. Chem. C, 2010, 114, 2070–2074 CrossRef CAS.
- S. Guo, S. Dong and E. Wang, Chem. – Eur. J., 2009, 15, 2416–2424 CrossRef CAS PubMed.
- Y. Y. Yu, S. Handa, T. Yajima and M. Futamata, Chem. Phys. Lett., 2013, 560, 49–54 CrossRef CAS.
- L. D. Ziegler, J. Raman Spectrosc., 1990, 21, 769–779 CrossRef CAS.
- D. A. Weitz, S. Garoff and T. J. Gramila, Opt. Lett., 1982, 7, 168–170 CrossRef CAS PubMed.
- P. B. Johnson and R. W. Christy, Phys. Rev. B: Solid State, 1972, 6, 4370–4379 CrossRef CAS.
- S. L. Kleinman, B. Sharma, M. G. Blaber, A.-I. Henry, N. Valley, R. G. Freeman, M. J. Natan, G. C. Schatz and R. P. Van Duyne, J. Am. Chem. Soc., 2013, 135, 301–308 CrossRef CAS PubMed.
- M. Z. Liu, T. W. Lee, S. K. Gray, P. Guyot-Sionnest and M. Pelton, Phys. Rev. Lett., 2009, 102, 107401 CrossRef PubMed.
- M. I. Stockman, in Surface-Enhanced Raman Scattering, ed. K. Kneipp, M. Moskovits and H. Kneipp, Springer, Berlin Heidelberg, 2006, vol. 103, ch. 3, pp. 47–65 Search PubMed.
- Y. Wang, W. Ji, H. M. Sui, Y. Kitahama, W. D. Ruan, Y. Ozaki and B. Zhao, J. Phys. Chem. C, 2014, 118, 10191–10197 CrossRef CAS.
- Y. Liu, H. Yuan, A. M. Fales and T. Vo-Dinh, J. Raman Spectrosc., 2013, 44, 980–986 CrossRef CAS.
- H. K. Park, S. B. Lee, K. Kim and M. S. Kim, J. Phys. Chem., 1990, 94, 7576–7580 CrossRef CAS.
- S. B. Lee, K. Kim and M. S. Kim, J. Raman Spectrosc., 1991, 22, 811–817 CrossRef CAS.
- R. J. Irving, L. Nelander and I. Wadsö, Acta Chem. Scand., 1964, 18, 769–787 CrossRef CAS.
- R. A. Alvarez-Puebla, D. S. Dos Santos and R. F. Aroca, Analyst, 2004, 129, 1251–1256 RSC.
- S. Handa, Y. Y. Yu and M. Futamata, Vib. Spectrosc., 2014, 72, 128–133 CrossRef CAS.
- A. K. Ojha, P. Donfack and A. Materny, J. Raman Spectrosc., 2012, 43, 1183–1190 CrossRef CAS.
- R. A. Alvarez-Puebla, E. Arceo, P. J. G. Goulet, J. J. Garrido and R. F. Aroca, J. Phys. Chem. B, 2005, 109, 3787–3792 CrossRef CAS PubMed.
- M. Kazanci, J. P. Schulte, C. Douglas, P. Fratzl, D. Pink and T. Smith-Palmer, Appl. Spectrosc., 2009, 63, 214–223 CrossRef CAS PubMed.
- V. Joseph, A. Matschulat, J. Polte, S. Rolf, F. Emmerling and J. Kneipp, J. Raman Spectrosc., 2011, 42, 1736–1742 CrossRef CAS.
- K. Kneipp, H. Kneipp and J. Kneipp, Chem. Sci., 2015, 6, 2721–2726 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Absorbance spectra of the nanosensors at different pH values; one-photon excited SERS spectra of pMBA at 532 nm; extracts of SEHRS and SERS spectra, showing pH-dependent band shifts; a table with band positions and assignments. See DOI: 10.1039/c5cp03844h |
| ‡ These authors contributed equally to this work. |
| This journal is © the Owner Societies 2015 |



