Do resonance-assisted intramolecular halogen bonds exist without a charge transfer and a σ-hole?†
Abstract
To analyze the properties and mechanisms of six types of intramolecular resonance-assisted halogen bonds (Br⋯O, Cl⋯O, F⋯O, Br⋯O, Cl⋯S and F⋯S), we have chosen the five-membered closed ring system X–C1R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3R2–C2R3
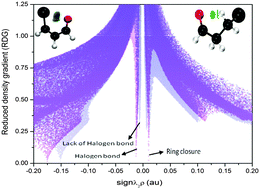
C3R2–C2R3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y (X = Br, Cl & F; Y = O & S) of unsaturated compounds with the substituents NO2, CH3 and H. A total of 78 structures were investigated by quantum chemical calculations at the MP2/aug-cc-pVTZ level of theory. A molecular electrostatic potential (MESP) map reveals that the cusp point of the σ-hole was not utilized but the belt point was used for all these intramolecular halogen-bonding interactions, indicating that all are electrostatic interactions. The halogen-bonding angle is below 100° with the strongest interactions. The value of the nucleus-independent chemical shift (NICS (1)) reflects the changes and efficiency of resonance in all structures with a long bond. The presence of all interactions was proved by the bond critical point (BCP) and analyzed through its electron density, Laplacian of electron density and ellipticity parameter. The linear-probability correlation between the difference of the sum of the van der Waals radius and the non-covalent bond length (∑vdW–L) and the electron density of the BCP was reported. 2D and 3D-NCI (non covalent interactions) plots show that halogen-bonding interactions are a mixed type of interactions with an attractive term. Natural bond orbital (NBO) analysis clearly indicates that the halogen bond lacks charge transfer and orbital overlapping through non-interacting lobes.
Y (X = Br, Cl & F; Y = O & S) of unsaturated compounds with the substituents NO2, CH3 and H. A total of 78 structures were investigated by quantum chemical calculations at the MP2/aug-cc-pVTZ level of theory. A molecular electrostatic potential (MESP) map reveals that the cusp point of the σ-hole was not utilized but the belt point was used for all these intramolecular halogen-bonding interactions, indicating that all are electrostatic interactions. The halogen-bonding angle is below 100° with the strongest interactions. The value of the nucleus-independent chemical shift (NICS (1)) reflects the changes and efficiency of resonance in all structures with a long bond. The presence of all interactions was proved by the bond critical point (BCP) and analyzed through its electron density, Laplacian of electron density and ellipticity parameter. The linear-probability correlation between the difference of the sum of the van der Waals radius and the non-covalent bond length (∑vdW–L) and the electron density of the BCP was reported. 2D and 3D-NCI (non covalent interactions) plots show that halogen-bonding interactions are a mixed type of interactions with an attractive term. Natural bond orbital (NBO) analysis clearly indicates that the halogen bond lacks charge transfer and orbital overlapping through non-interacting lobes.

 Please wait while we load your content...
Please wait while we load your content...