 Open Access Article
Open Access ArticleSpectroscopic evidence of ‘jumping and pecking’ of cholinium and H-bond enhanced cation–cation interaction in ionic liquids†
Anne
Knorr
a,
Koichi
Fumino
a,
Anne-Marie
Bonsa
a and
Ralf
Ludwig
*ab
aUniversität Rostock, Institut für Chemie, Abteilung für Physikalische Chemie, Dr.-Lorenz-Weg 1, 18059 Rostock, Germany. E-mail: ralf.ludwig@uni-rostock.de; Fax: +49 381 498 6524; Tel: +49 381 498 6517
bLeibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
First published on 11th August 2015
Abstract
The subtle energy-balance between Coulomb-interaction, hydrogen bonding and dispersion forces governs the unique properties of ionic liquids. To measure weak interactions is still a challenge. This is in particular true in the condensed phase wherein a melange of different strong and directional types of interactions is present and cannot be detected separately. For the ionic liquids (2-hydroxyethyl)-trimethylammonium (cholinium) bis(trifluoro-methylsulfonyl)amide and N,N,N-trimethyl-N-propylammonium bis(trifluoromethylsulfonyl)amide which differ only in the 2-hydroxyethyl and the propyl groups of the cations, we could directly observe distinct vibrational signatures of hydrogen bonding between the cation and the anion indicated by ‘jumping and pecking’ motions of cholinium. The assignment could be confirmed by isotopic substitution H/D at the hydroxyl group of cholinium. For the first time we could also find direct spectroscopic evidence for H-bonding between like-charged ions. The repulsive Coulomb interaction between the cations is overcome by cooperative hydrogen bonding between the 2-hydroxyethyl functional groups of cholinium. This H-bond network is reflected in the properties of protic ionic liquids (PILs) such as viscosities and conductivities.
Investigating non-covalent interactions in liquids is still a challenge.1–5 This is in particular true for ionic liquids, where a subtle energy balance between Coulomb interaction, hydrogen bonding and dispersion forces results in unique properties.4,5 Although the Coulomb interaction is the dominant intermolecular interaction, hydrogen bonding and dispersion-forces may become crucial for the structure and dynamics of ionic liquids. We could show recently for imidazolium ionic liquids that the enhanced cation–anion interaction due to hydrogen bonding is indicated by frequency shifts to higher wave numbers in the far infrared.6,7 But only for protic ionic liquids we could observe distinguished vibrational bands which do not overlap with vibrational signatures of other inter- or intramolecular motions.8,9 Whereas cation–anion pairing via hydrogen bonding is well characterized, cation–cation interaction is not reported for protic ionic liquids yet. This rare phenomenon has been observed for aqueous salt solutions K/CsBr,10 for guanidinium ions in water,11 and in the micellization of tetraalkylammonium surfactants.12 Mele could measure Nuclear Overhauser Effect (NOE) contacts between protons of imidazolium cations in aprotic ionic liquids.13 There was some information about the distance but no evidence for the type and the strength of interaction. Most recently, Gamrad et al. reported self-association of simple organic cations based on hydrogen bonding. Cation–cation pairing was detected in crystal structures as observed in the X-ray structure.14 To the best of our knowledge spectroscopic evidence for directional interactions between ions of like charge in protic ionic liquids has not been reported so far.
Here we have chosen cholinium-based ionic liquids because they provide functional groups outside of the cation charge center. Thus we expected to observe two different types of intermolecular interaction including H-bond formation between the cation and the anion and possibly between ions of like charge. All relevant spectroscopic signatures should be detectable in far and mid infrared spectra. Important questions can be addressed appropriately. What do these H-bond motions look like? Is H-bond formation restricted to cation–anion pairs or is it possible between ions of like charge? And finally, how is this structural and dynamic behaviour reflected in properties such as melting temperatures, viscosities and conductivities?
First, we tested the ionic liquids (2-hydroxyethyl)-trimethylammonium (cholinium) bis(trifluoromethylsulfonyl)amide, [Ch][NTf2] (I) and N,N,N-trimethyl-N-propylammonium bis(trifluoromethylsulfonyl)amide, [TMPA][NTf2] (II) in the low frequency range between 10 and 250 cm−1. For both ILs the anion is the same and only the cations differ in the 2-hydroxyethyl group for I and the propyl group for II. As shown in Fig. 1, the far infrared spectra look similar except for the vibrational band at 176 cm−1 for the cholinium-based IL I. This well distinguished vibrational signature can only be related to the intermolecular vibrational mode between the hydroxyl group of the 2-hydroxyethyl trimethylammonium and the oxygen of the NTf2 anion. In IL II the hydroxyl group is replaced by a methyl group which does not allow hydrogen bonding to the anion. Consequently such a vibrational band cannot be observed for IL II.
Dispersion-corrected density functional calculations (DFT-D3) for ion-pairs of [Ch][NTf2] support this finding and provide ‘visualization’ of this vibrational mode.15–17 As shown in Scheme 1 the vibrational band at 176 cm−1 shows ‘pecking’ of the cholinium cation along the OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bond towards the NTf2 anion. The other important mode covering the range between 80 cm−1 and 100 cm−1 only slightly differs for both ILs. This broad band is typical for all ILs and describes the unspecific cation–anion interaction.6–9 In our case this motion can be regarded as cation ‘jumping’ on the anion as shown in Scheme 1. This is due to the significantly larger mass of the anion (280 a.u.) compared to that of the cation (104 a.u). That the ‘pecking’ mode is about 80 cm−1 higher in frequency is rather due to lower reduced mass than increased force constant due to stronger interaction.
S hydrogen bond towards the NTf2 anion. The other important mode covering the range between 80 cm−1 and 100 cm−1 only slightly differs for both ILs. This broad band is typical for all ILs and describes the unspecific cation–anion interaction.6–9 In our case this motion can be regarded as cation ‘jumping’ on the anion as shown in Scheme 1. This is due to the significantly larger mass of the anion (280 a.u.) compared to that of the cation (104 a.u). That the ‘pecking’ mode is about 80 cm−1 higher in frequency is rather due to lower reduced mass than increased force constant due to stronger interaction.
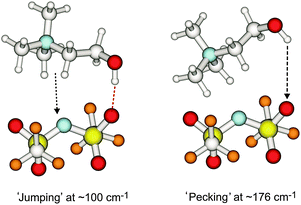 | ||
| Scheme 1 Illustration of the observed and calculated intermolecular vibrational modes at 100 and 176 cm−1, called ‘jumping’ and ‘pecking’ motion of cholinium on the NTf2− anion in IL I. | ||
Additional experimental evidence for the ‘pecking’ mode, we expected by H/D exchange at the hydroxyl group due to increasing molecular weight. The DFT-D3 calculations suggest a redshift of about 3 cm−1 by isotope exchange H/D at the OH-group (see the ESI†). Such a shift can be observed only if the 2-hydroxyethyl group but not the whole cation is involved in this intermolecular motion. Then the mass of the 2-hydroxyethyl group increases from 45 to 46 units and should result in a detectable frequency shift. And indeed, the measured vibrational band is shifted from 176 cm−1 down to 173 cm−1 as expected for such a small change in the reduced mass and remaining strength in interaction. This shift is shown in Fig. 2 for the FIR spectra of the protonated and deuterated species as a function of temperature between 313 and 353 K. Because the spectral resolution is in the order of 2 cm−1, the shift can be slightly larger or smaller over the whole temperature range but indicates a clear redshift of 3 cm−1 on average.
The ‘pecking’ motion along the OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bond between cholinium and NTf2 is also reflected in the mid infrared spectra. In Fig. 3 the OH stretching region is shown as a function of temperature between 303 and 353 K. All spectra could be properly decomposed into four contributions at 3431 cm−1, 3474 cm−1, 3541 cm−1 and 3624 cm−1 (also see the ESI†). The dominant OH band at 3541 cm−1 indicates a relatively weak hydrogen bond between the cation and the anion and is related to the ‘pecking’ band in the FIR spectrum at 176 cm−1. It is not more redshifted than the OH stretching frequencies of H-bonded alcohol dimers.18,19 This mode loses intensity as a function of temperature for the benefit of the band at 3624 cm−1 indicating a quasi-free OH vibrational mode as known from the literature.20,21 The cation–anion interaction and its temperature behavior has been recently discussed for choline systems supported by molecular dynamics simulations.22,23
S hydrogen bond between cholinium and NTf2 is also reflected in the mid infrared spectra. In Fig. 3 the OH stretching region is shown as a function of temperature between 303 and 353 K. All spectra could be properly decomposed into four contributions at 3431 cm−1, 3474 cm−1, 3541 cm−1 and 3624 cm−1 (also see the ESI†). The dominant OH band at 3541 cm−1 indicates a relatively weak hydrogen bond between the cation and the anion and is related to the ‘pecking’ band in the FIR spectrum at 176 cm−1. It is not more redshifted than the OH stretching frequencies of H-bonded alcohol dimers.18,19 This mode loses intensity as a function of temperature for the benefit of the band at 3624 cm−1 indicating a quasi-free OH vibrational mode as known from the literature.20,21 The cation–anion interaction and its temperature behavior has been recently discussed for choline systems supported by molecular dynamics simulations.22,23
 | ||
| Fig. 3 (a) Mid infrared spectrum in the OH stretching region of the ionic liquid (2-hydroxyethyl)-trimethyl-ammonium bis(trifluoromethylsulfonyl)amide as a function of temperature. The red arrow indicates the decreasing intensities of the vibrational bands slightly above 3400 cm−1 with increasing temperature. (b) The spectra can be deconvoluted into vibrational bands at 3431 cm−1, 3474 cm−1, 3541 cm−1 and 3624 cm−1. The most intense high frequency band (red) can be assigned to the OH stretching mode along the H-bond from the cation to the anion. The low frequency bands (blue and purple) indicate cation–cation hydrogen bonding as illustrated in Scheme 2b. | ||
The most interesting vibrational bands appear in the low frequency edge of the spectra at 3431 cm−1 and 3474 cm−1 (see Fig. 3b). They are redshifted about 110 cm−1 and 67 cm−1 relative to the dominant OH band at 3541 cm−1. DFT-D3 calculations suggest that these vibrational signatures indicate hydrogen bonding between OH groups of the two cations. Whereas the dominant OH mode results from structures with single hydrogen bonds OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S between the cation and the anion (see Scheme 2a), these redshifted vibrational bands can be assigned to additional cation–cation H-bond interaction as shown in Scheme 2b. For the first time we report spectroscopic evidence for directional cation–cation interaction via hydrogen bonding in ILs. Recently, Mele reported NOE contacts between protons of imidazolium cations in ILs, but no specific, well characterized interactions could be observed.13
S between the cation and the anion (see Scheme 2a), these redshifted vibrational bands can be assigned to additional cation–cation H-bond interaction as shown in Scheme 2b. For the first time we report spectroscopic evidence for directional cation–cation interaction via hydrogen bonding in ILs. Recently, Mele reported NOE contacts between protons of imidazolium cations in ILs, but no specific, well characterized interactions could be observed.13
It is interesting to note that the vibrational band describing the OH⋯OH interaction between two choliniums is redshifted about 110 cm−1 in comparison to that of the OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S interaction between the cation and the anion. This frequency shift is similar to the 112 cm−1 shift observed for ethanol dimers in carbon tetrachloride solutions. Both spectra are shown in Fig. 4. If we bring the OH band of the ethanol monomer in line with the main OH band of cholinium at 3541 cm−1 (as indicated by the green dotted lines in Fig. 4), we observe the same frequency shifts of the OH⋯OH bands describing the cation–cation and the ethanol dimer interaction. Although we are dealing with ILs, the hydrogen bond strength is in the order of that found for molecular liquids such as alcohols.18–21
S interaction between the cation and the anion. This frequency shift is similar to the 112 cm−1 shift observed for ethanol dimers in carbon tetrachloride solutions. Both spectra are shown in Fig. 4. If we bring the OH band of the ethanol monomer in line with the main OH band of cholinium at 3541 cm−1 (as indicated by the green dotted lines in Fig. 4), we observe the same frequency shifts of the OH⋯OH bands describing the cation–cation and the ethanol dimer interaction. Although we are dealing with ILs, the hydrogen bond strength is in the order of that found for molecular liquids such as alcohols.18–21
However, the most surprising finding here is the existence of hydrogen bonding not only between the anion and the cation but also among cations themselves. To the best of our knowledge this is the first example of H-bonding between ions of like charge overcoming significant repulsive electrostatic forces. This interpretation is supported by DFT-D3 calculated frequencies of such species. The temperature behavior of the OH vibrational bands is a further proof. The intensity of the OH⋯OH vibrational band decreases with increasing temperature (Fig. 3a). Obviously the cation–cation H-bonds break upon introducing thermal energy. The DFT-D3 calculations show that this is mainly for entropic reasons. The cation–cation hydrogen bonds result in larger aggregates which are entropically unfavorable (Scheme 2b; see also the ESI†).
The DFT-D3 calculations also suggest that the cation–cation OH⋯OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S interaction is possible due to cooperative effects.20,21 Charge from the anion oxygen is donated to the OH antibond of the first cation. The larger negative charge at this oxygen can now be transferred to the OH antibond of the second cation further enhancing hydrogen bonding. In this way the short-range donor–acceptor covalent forces can overcome the strong long-range electrostatic repulsive forces as expected for ions of like charge. These features can be rationalized in the framework of the natural bond orbital (NBO) analysis.24,25 Recently, Weinhold and Klein characterized a surprising new class of H-bonded complexes comprising ions of like charge.26,27 These species exhibited appreciable kinetic stability and typical structural and spectroscopic signatures of hydrogen bonding, despite strong repulsive electrostatic forces. This prediction from quantum mechanical calculations and NBO analysis seems to be supported here experimentally. The repulsive electrostatic forces are overcome by directional, cooperative H-bonds indicated by characteristic redshift in the OH stretch region. NBO analysis shows typical strong no → σ*OH donor–acceptor interaction, corresponding to second order stabilization energies E(2)n→σ* = 20.7 kcal mol−1 and estimated total charge transfers of qTC = 0.0393 e for the (S)O⋯HO H-bond as well as 25.45 kcal mol−1 and 0.0451 e for the enhanced O⋯HO H-bond, respectively. The related values for the H-bond in the ethanol dimers are calculated to be 14.28 kcal mol−1 and 0.0251 e. Both the stabilization energies and the charge transfers reflect the order of the measured IR redshifts as shown in Fig. 3b (see also the ESI†). The stabilization energy, the total charge transfer and the frequency shift are similar for the cation–cation and the molecule–molecule H-bonds as reflected in the infrared spectra.
S interaction is possible due to cooperative effects.20,21 Charge from the anion oxygen is donated to the OH antibond of the first cation. The larger negative charge at this oxygen can now be transferred to the OH antibond of the second cation further enhancing hydrogen bonding. In this way the short-range donor–acceptor covalent forces can overcome the strong long-range electrostatic repulsive forces as expected for ions of like charge. These features can be rationalized in the framework of the natural bond orbital (NBO) analysis.24,25 Recently, Weinhold and Klein characterized a surprising new class of H-bonded complexes comprising ions of like charge.26,27 These species exhibited appreciable kinetic stability and typical structural and spectroscopic signatures of hydrogen bonding, despite strong repulsive electrostatic forces. This prediction from quantum mechanical calculations and NBO analysis seems to be supported here experimentally. The repulsive electrostatic forces are overcome by directional, cooperative H-bonds indicated by characteristic redshift in the OH stretch region. NBO analysis shows typical strong no → σ*OH donor–acceptor interaction, corresponding to second order stabilization energies E(2)n→σ* = 20.7 kcal mol−1 and estimated total charge transfers of qTC = 0.0393 e for the (S)O⋯HO H-bond as well as 25.45 kcal mol−1 and 0.0451 e for the enhanced O⋯HO H-bond, respectively. The related values for the H-bond in the ethanol dimers are calculated to be 14.28 kcal mol−1 and 0.0251 e. Both the stabilization energies and the charge transfers reflect the order of the measured IR redshifts as shown in Fig. 3b (see also the ESI†). The stabilization energy, the total charge transfer and the frequency shift are similar for the cation–cation and the molecule–molecule H-bonds as reflected in the infrared spectra.
After all spectral analyses one may ask whether the cation–anion and cation–cation H-bonding is reflected in thermodynamic and transport properties of the ILs? Comparing ILs I and II it can be observed that H-bonding in I results in higher melting temperatures, larger viscosities and lower electrical conductivities as shown in Table 1. In an earlier study of imidazolium-based aprotic ionic liquids we claimed that H-bonding can lead to lower melting temperatures and higher viscosities due to the preformation of ion-pairs.6,7 That we find the opposite behaviour here could be related to the overall H-bond network formation. Significant attractive cation–cation interaction leads to the formation of larger aggregates resulting in increased viscosities.
| IL | T m/°C | η/mPa s | σ/S cm−1 |
|---|---|---|---|
| I [Ch][NTf2] | 27 | 41.9 | 3.34 |
| II [TMPA][NTf2] | 19 | 28.9 | 4.68 |
We could show for ionic liquids that different types of intermolecular interaction between the cation and the anion are possible. The typical non-directional interaction results in a broad and unspecific vibrational band at 100 cm−1, whereas the H-bond gives a distinct vibrational band at 176 cm−1. These motions resemble ‘jumping’ and ‘pecking’ of the cation on the anion. For the first time we could provide evidence for hydrogen bonding between ions of like charge. In principle the hydroxyl groups of two choliniums can form cooperative H-bonds OH⋯OH⋯OS similar to those in alcohol dimers. The attractive cation–cation interaction results in interesting H-bond networks, wherein not only cations and anions are connected as known for other ILs. Finally we could show that the enhanced H-bond network results in increasing melting temperatures and viscosities as well as decreasing conductivities. In future studies it would be shown how the formation of cation–cation pairing depends on the type of cation and on the interaction strength of the chosen anions. These kinds of studies are currently going on in our laboratories.
This research was supported by the DFG Priority Programme SPP 1807 and partly funded by the DFG Collaborative Research Center SFB 652. The author gratefully acknowledges the support from the Leibniz-Institut for Catalysis.
Notes and references
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- H. Weingärtner, Angew. Chem., 2008, 120, 664–682 ( Angew. Chem., Int. Ed. , 2008 , 47 , 654–670 ) CrossRef PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- K. Fumino and R. Ludwig, J. Mol. Liq., 2014, 192, 94–102 CrossRef CAS PubMed.
- K. Fumino, S. Reimann and R. Ludwig, Phys. Chem. Chem. Phys., 2014, 40, 21903–21929 RSC.
- K. Fumino, A. Wulf and R. Ludwig, Angew. Chem., 2008, 120, 8859–8862 ( Angew. Chem., Int. Ed. , 2008 , 47 , 8731–8734 ) CrossRef PubMed.
- K. Fumino, T. Peppel, M. Geppert-Rybczynska, D. H. Zaitsau, J. K. Lehmann, S. P. Verevkin, M. Köckerling and R. Ludwig, Phys. Chem. Chem. Phys., 2011, 13, 14064–14075 RSC.
- K. Fumino, E. Reichert, K. Wittler, R. Hempelmann and R. Ludwig, Angew. Chem., 2012, 124, 6340–6344 ( Angew. Chem., Int. Ed. , 2012 , 51 , 6236–6240 ) CrossRef PubMed.
- K. Fumino, V. Fossog, K. Wittler, R. Hempelmann and R. Ludwig, Angew. Chem., 2013, 125, 2425–2429 ( Angew. Chem., Int. Ed. , 2013 , 52 , 2368–2372 ) CrossRef PubMed.
- M. Holz and K. J. Patil, Ber. Bunsen-Ges., 1991, 95, 107–113 CrossRef CAS PubMed.
- O. Shih, A. H. England, G. C. Dallinger, J. W. Smith, K. C. Duffey, R. C. Cohen, D. Prendergast and R. J. Saykally, J. Chem. Phys., 2013, 139, 035104 CrossRef PubMed.
- M. Benrraou, B. L. Bales and R. Zana, J. Phys. Chem. B, 2003, 107, 13432–13440 CrossRef CAS , and references therein.
- A. Mele, G. Romanò, M. Giannone, E. Ragg, G. Fronza, G. Raos and V. Marcon, Angew. Chem., Int. Ed., 2006, 45, 1123–1126 ( Angew. Chem. , 2006 , 118 , 1141–1144 ) CrossRef CAS PubMed.
- W. Gamrad, A. Dreier, R. Goddrad and K.-R. Pörschke, Angew. Chem., 2015, 127, 4564–4569 ( Angew. Chem., Int. Ed. , 2015 , 54 , 4482–4487 ) CrossRef PubMed.
- M. J. Frisch, et al., Gaussian 09 (Revision B.01) Search PubMed, see the ESI†.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Ehrlich, J. Moellmann, W. Reckien, T. Bredow and S. Grimme, ChemPhysChem, 2011, 12, 3414–3420 CrossRef CAS PubMed.
- K. M. Murdoch, T. D. Ferris, J. C. Wright and T. C. Farrar, J. Chem. Phys., 2002, 116, 5717 CrossRef CAS PubMed.
- F. Huisken, A. Kulcke, C. Laush and J. Lisy, J. Chem. Phys., 1991, 95, 3924–3929 CrossRef CAS PubMed; M. Huelsekopf and R. Ludwig, J. Mol. Liq., 2002, 98–99, 163–171 CrossRef.
- R. Ludwig, F. Weinhold and T. C. Farrar, Mol. Phys., 1999, 97, 465–477 CrossRef CAS PubMed.
- R. Ludwig, Phys. Chem. Chem. Phys., 2002, 4, 5481–5487 RSC.
- A. J. L. Costa, M. R. C. Soromenho, K. Shimizu, J. M. S. S. Esperanca, J. N. Canongia Lopes and L. P. N. Rebelo, RSC Adv., 2013, 3, 10262–10271 RSC.
- A. J. L. Costa, M. R. C. Soromenho, K. Shimizu, I. M. Marrucho, J. M. S. S. Esperanca, J. N. Canongia Lopes and L. P. N. Rebelo, J. Phys. Chem. B, 2012, 116, 9186–9195 CrossRef CAS PubMed.
- E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef.
- F. Weinhold and C. R. Landis, Valency and Bonding A Natural Bond Orbital Donor-Acceptor Perspective, Cambridge, University Press, Cambridge, 2005 Search PubMed.
- (a) F. Weinhold and R. A. Klein, Angew. Chem., 2014, 126, 11396–11399 ( Angew. Chem., Int. Ed. , 2014 , 53 , 11214–11217 ) CrossRef PubMed; (b) Addendum: F. Weinhold and R. A. Klein, Angew. Chem., 2014, 126, 13207 ( Angew. Chem., Int. Ed. , 2014 , 53 , 12992 ) CrossRef PubMed.
- F. Weinhold and R. A. Klein, Angew. Chem., 2015, 127, 2636–2638 ( Angew. Chem., Int. Ed. , 2015 , 54 , 2600–2602 ) CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cp03412d |
| This journal is © the Owner Societies 2015 |




