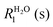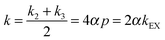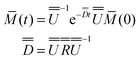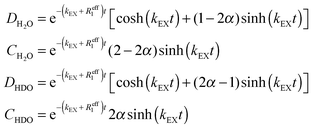Open Access Article
Open Access ArticleChallenges in preparing, preserving and detecting para-water in bulk: overcoming proton exchange and other hurdles
Daniele
Mammoli
*a,
Nicola
Salvi
a,
Jonas
Milani
a,
Roberto
Buratto
a,
Aurélien
Bornet
a,
Akansha Ashvani
Sehgal
bcd,
Estel
Canet
abcd,
Philippe
Pelupessy
bcd,
Diego
Carnevale
e,
Sami
Jannin
af and
Geoffrey
Bodenhausen
*abcd
aInstitut des Sciences et Ingéniérie Chimiques, Ecole Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland. E-mail: daniele.mammoli@epfl.ch; geoffrey.bodenhausen@epfl.ch
bÉcole Normale Supérieure-PSL Research University, Département de Chimie, 24 rue Lhomond, 75005 Paris, France
cSorbonne Universités, UPMC Univ. Paris 06, LBM, 4 place Jussieu, 75005 Paris, France
dCNRS, UMR 7203 LBM, 75005 Paris, France
eNeuchâtel Platform of Analytical Chemistry (NPAC), Institut de Chimie, Université de Neuchâtel, 2000 Neuchâtel, Switzerland
fBruker BioSpin AG, Industriestrasse 26, 8117 Fällanden, Switzerland
First published on 28th August 2015
Abstract
Para-water is an analogue of para-hydrogen, where the two proton spins are in a quantum state that is antisymmetric under permutation, also known as singlet state. The populations of the nuclear spin states in para-water are believed to have long lifetimes just like other Long-Lived States (LLSs). This hypothesis can be verified by measuring the relaxation of an excess or a deficiency of para-water, also known as a “Triplet–Singlet Imbalance” (TSI), i.e., a difference between the average population of the three triplet states T (that are symmetric under permutation) and the population of the singlet state S. In analogy with our recent findings on ethanol and fumarate, we propose to adapt the procedure for Dissolution Dynamic Nuclear Polarization (D-DNP) to prepare such a TSI in frozen water at very low temperatures in the vicinity of 1.2 K. After rapid heating and dissolution using an aprotic solvent, the TSI should be largely preserved. To assess this hypothesis, we studied the lifetime of water as a molecular entity when diluted in various solvents. In neat liquid H2O, proton exchange rates have been characterized by spin-echo experiments on oxygen-17 in natural abundance, with and without proton decoupling. One-dimensional exchange spectroscopy (EXSY) has been used to study proton exchange rates in H2O, HDO and D2O mixtures diluted in various aprotic solvents. In the case of 50 mM H2O in dioxane-d8, the proton exchange lifetime is about 20 s. After dissolving, one can observe this TSI by monitoring intensities in oxygen-17 spectra of H2O (if necessary using isotopically enriched samples) where the AX2 system comprising a “spy” oxygen A and two protons X2 gives rise to binomial multiplets only if the TSI vanishes. Alternatively, fast chemical addition to a suitable substrate (such as an activated aldehyde or ketone) can provide AX2 systems where a carbon-13 acts as a spy nucleus. Proton signals that relax to equilibrium with two distinct time constants can be considered as a hallmark of a TSI. We optimized several experimental procedures designed to preserve and reveal dilute para-water in bulk.
Introduction
Although water is a fundamental constituent of our biosphere, characterization of some of its most basic properties remains a formidable challenge. Most applications of magnetic resonance imaging (MRI), both in vitro and in vivo, are based on observing the magnetization of the two hydrogen nuclei of water. This is actually limited to the observation of ortho-water. If the populations of the three symmetrical states deviate from Boltzmann equilibrium, they rapidly recover through longitudinal spin–lattice relaxation with a time constant T1, which is typically on the order of 10 s in neat water. So far, neither NMR nor MRI has been able to exploit the properties of the invisible singlet state S0, also known as para-water, by analogy to para-hydrogen.1,2 The following linear combination of populations:| ⅓[|T+1〉〈T+1| + |T0〉〈T0| + |T−1〉〈T−1|] − |S0〉〈S0| | (1) |
is equivalent to a “Triplet–Singlet Imbalance” (henceforth TSI), in analogy to the expression coined by Meier et al.3 for the “A/E imbalance” in 13CH3 groups, which refers to a population imbalance between symmetric and antisymmetric states belonging to the irreducible representations A and E of methyl groups. An A/E imbalance can be induced by cooling down to ca. 1 K molecules such as γ-picoline that contain methyl groups characterized by very low rotational barriers.3–8 In the high-temperature approximation, i.e., at spin temperatures above a few degree Kelvin, the distribution between para- and ortho-water is given by the ratio 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. If this ratio is perturbed, as we shall demonstrate in this paper, the resulting TSI in eqn (1) should have a lifetime that might be much longer than the spin–lattice relaxation time (TTSI > T1). This is analogous to molecules that contain two magnetically inequivalent protons that can sustain a Long-Lived State (LLS).9 Such an LLS can preserve spin order over periods that are much longer than T1. In 12CH2 groups, for instance, we have shown that one can have lifetimes with ratios TTSI/T1 > 36.10 The LLS can be prepared by several NMR techniques at room temperature10,11 or by DNP at low temperature.12 Optimization of nearly-symmetric molecules comprising pairs of 13C nuclei has led to very long lifetimes in solution that can exceed one hour at room temperature.13 Another intriguing feature of the LLS is that they can be used to improve the sensitivity of drug screening experiments.14–16 In the past, several studies have focused on the isolation of para-water and the characterization of its long-lived behavior.17 In crystal water trapped in gypsum (CaSO4·2H2O), Pake found evidence of isolated pairs of protons.18 At T < 100 K, the water molecules trapped in gypsum crystals cannot flip around their two-fold symmetry axis. As a result, the two protons may be at unequal distances from other protons belonging to remote hydration water molecules. Thus the two protons may experience different intermolecular dipolar couplings, and their magnetic equivalence can be lifted. It is therefore possible to populate the antisymmetric state. Eisendrath, Stone and Jeener19,20 characterized para-water in solid gypsum. More recently, the separation of ortho- and para-water has been achieved in molecular beams travelling through inhomogeneous magnetic fields,21 where a beam of ortho-water can be deflected and refocused in the manner of the Stern–Gerlach experiment, or in inhomogeneous electric fields.22 These methods can produce a large TSI but only for very small quantities of water. Isomer enrichment of H2O in bulk, by means of absorption in column chromatography, has been proposed23 and later challenged.24 In other studies, the interconversion between para- and ortho-isomers was achieved by isolating water molecules in frozen inert gases25–27 or by trapping them in C60 cages.28 In the latter case, the conversion has been monitored by a combination of infrared spectroscopy, inelastic neutron scattering and cryo-MAS NMR spectroscopy.29 This allows one to study ortho–para conversion rates30 of isolated water molecules. However, the confinement in C60 cages prevents one from monitoring interactions with surrounding molecules. In this work we shall discuss the possibility of preparing samples of non-confined water characterized by a significant TSI at concentrations on the order of a few mM. Our approach is similar to our strategy for preparing hyperpolarized para-ethanol31 and para-fumarate.32
3. If this ratio is perturbed, as we shall demonstrate in this paper, the resulting TSI in eqn (1) should have a lifetime that might be much longer than the spin–lattice relaxation time (TTSI > T1). This is analogous to molecules that contain two magnetically inequivalent protons that can sustain a Long-Lived State (LLS).9 Such an LLS can preserve spin order over periods that are much longer than T1. In 12CH2 groups, for instance, we have shown that one can have lifetimes with ratios TTSI/T1 > 36.10 The LLS can be prepared by several NMR techniques at room temperature10,11 or by DNP at low temperature.12 Optimization of nearly-symmetric molecules comprising pairs of 13C nuclei has led to very long lifetimes in solution that can exceed one hour at room temperature.13 Another intriguing feature of the LLS is that they can be used to improve the sensitivity of drug screening experiments.14–16 In the past, several studies have focused on the isolation of para-water and the characterization of its long-lived behavior.17 In crystal water trapped in gypsum (CaSO4·2H2O), Pake found evidence of isolated pairs of protons.18 At T < 100 K, the water molecules trapped in gypsum crystals cannot flip around their two-fold symmetry axis. As a result, the two protons may be at unequal distances from other protons belonging to remote hydration water molecules. Thus the two protons may experience different intermolecular dipolar couplings, and their magnetic equivalence can be lifted. It is therefore possible to populate the antisymmetric state. Eisendrath, Stone and Jeener19,20 characterized para-water in solid gypsum. More recently, the separation of ortho- and para-water has been achieved in molecular beams travelling through inhomogeneous magnetic fields,21 where a beam of ortho-water can be deflected and refocused in the manner of the Stern–Gerlach experiment, or in inhomogeneous electric fields.22 These methods can produce a large TSI but only for very small quantities of water. Isomer enrichment of H2O in bulk, by means of absorption in column chromatography, has been proposed23 and later challenged.24 In other studies, the interconversion between para- and ortho-isomers was achieved by isolating water molecules in frozen inert gases25–27 or by trapping them in C60 cages.28 In the latter case, the conversion has been monitored by a combination of infrared spectroscopy, inelastic neutron scattering and cryo-MAS NMR spectroscopy.29 This allows one to study ortho–para conversion rates30 of isolated water molecules. However, the confinement in C60 cages prevents one from monitoring interactions with surrounding molecules. In this work we shall discuss the possibility of preparing samples of non-confined water characterized by a significant TSI at concentrations on the order of a few mM. Our approach is similar to our strategy for preparing hyperpolarized para-ethanol31 and para-fumarate.32
Methodology
Our approach involves three consecutive steps.Step 1 – preparing a TSI
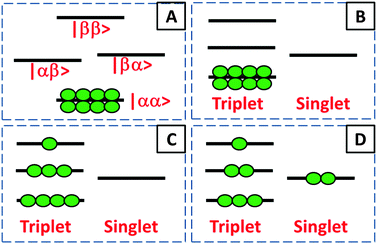
A flow of populations between the triplet and singlet energy levels in water cannot be induced by intramolecular dipole–dipole (DD) interactions between the two protons belonging to the same water molecule, although it may be induced by intermolecular dipole–dipole (DD) interactions19,20 or by the proton chemical shift anisotropy (CSA) in the condensed phase. In liquid H2O diluted in D2O at 300 K, the CSA has been determined to be 28 ppm.33 In gypsum, the proton CSA of the (hopping) water molecules has been determined to be 10 ppm at 300 K.34 The proton CSA of the hopping water molecules in Ba(ClO3)2·H2O has also been determined to be 10 ppm at 300 K.35 In glassy frozen solutions, the symmetry of the two protons in each H2O molecule is broken by the anisotropy of the chemical shifts, except for some particular orientations, so that the singlet state |S0〉 is mixed with the central triplet state |T+1〉. Depending on the coupling between the rotational and Zeeman energy levels, two situations can occur. If the energy levels are primarily determined by the rotational quantum numbers, as in the gas phase36 or C60 cages (where the rotational levels typical for the gas phase remain a good approximation), the singlet state S0 has the lowest energy. On the other hand, if rotational quantization can be neglected, the ground state is |αα〉 = |T+1〉 which belongs to the triplet manifold. In our samples, the rotation of water is believed to be hindered by hydrogen bonding with other water and/or solvent molecules. Thus, when H2O molecules are diluted in a deuterated aprotic solvent, doped with a radical and frozen at low temperatures in a high magnetic field, DNP can be used to achieve a TSI. The ESR transitions of the radical can be saturated by microwave irradiation to populate mostly the |αα〉 = |T+1〉 state (Fig. 1A). During dissolution, the magnetic equivalence of the two protons in each H2O molecule is restored. Hence, our strategy should lead to an excess of the average population of the three triplet states compared to the population of the singlet state (Fig. 1B). This amounts to a TSI. If its life-time is longer than T1, this may be considered as the hallmark of para-water.Step 2 – protecting the TSI during transfer
After rapid heating of the sample by injection of a hot aprotic solvent, the sample can be transferred37 to an NMR or MRI system. Longitudinal T1 relaxation in a few seconds leads to the return of the triplet manifold to Boltzmann equilibrium at room temperature (Fig. 1C). However, T1 relaxation does not affect the TSI, so that the singlet state remains depleted. On a longer time-scale TTSI, the populations of the triplet and singlet states will return to their Boltzmann equilibrium (Fig. 1D). In the dissolution step, all relaxation mechanisms that could reduce TTSI must be kept under control. The radicals can be eliminated by chemical reduction with scavengers such as ascorbate.38 Alternatively, porous solids containing covalently bound radicals can be filtered after dissolution.39 Spin rotation (SR) is another mechanism that could cause losses of the TSI. SR is due to the coupling between the nuclear spins and the molecular magnetic dipole induced by the electric dipole moment of the H2O molecule as it undergoes rotational diffusion. The SR mechanism of ortho–para conversion has been intensively studied.40,41 Indeed, in the gas phase,42,43 the longitudinal relaxation times were found to be on the order of T1 = 20 ms near 0.1 MPa and 373 K at 800 MHz. We believe however that SR is not an efficient mechanism for H2O in the condensed phase where collisions on the atomic scale should make SR ineffective. This should also be the case when the rotation of a H2O molecule is hindered because it is trapped in a cage of an aprotic solvent. Finally, one should pay attention to the exchange of protons between different water molecules. In other words, the lifetime τEX = 1/kEX of a water molecule as a molecular entity has to be longer than the lifetime of the TSI. Since the latter lifetime is unknown, the best option is to slow down the proton exchange as much as possible. In pure water the lifetime of proton exchange has been assessed to be τEX ≈ 1 ms by studying either linewidths in proton spectra44 or intensities of 17O lines with and without proton decoupling.45,46 However, it can be shown by EXSY47 that dilution in aprotic solvents can effectively slow down the exchange of protons and thus extend τEX. This approach should help to preserve the TSI and thus the lifetime of para-water.Step 3 – detecting TSI relaxation
To detect the relaxation of the TSI, at least four distinct approaches can be used. (i) Direct detection by infrared (IR) spectroscopy relies on the fact that ortho- and para-water give rise to distinct IR absorption bands in the gas phase.29,36 However, in the condensed phase, in particular, when water is diluted in aprotic solvents, we found that the IR signatures are difficult to identify. (ii) Direct detection by multiplet effects in oxygen-17 NMR. In thermal equilibrium, the oxygen-17 signal of H2O shows a normal binomial 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 triplet. If, however, one is able to populate a TSI, non-binomial multiplets could be observed like in para-ethanol.31 (iii) If the proton signals relax back to equilibrium with two different time constants this would be a hallmark of ortho–para conversion. (iv) Long-lived water can be revealed indirectly by chemical addition onto a suitable substrate such as an activated aldehyde or ketone, monitored by 1H or 13C NMR. For this strategy to be successful, the reactivity of water molecules has to fulfill three requirements that are partly contradictory: (a) the water must be sufficiently diluted so that intermolecular 1H exchange is slowed down; (b) the rate of the chemical addition that is used to reveal the presence of para-water must be faster than the relaxation of the TSI; and (c) the two protons that are added onto the substrate must stem from the same water molecule. Our kinetic measurements show that, under suitable conditions, these requirements may indeed be satisfied.
1 triplet. If, however, one is able to populate a TSI, non-binomial multiplets could be observed like in para-ethanol.31 (iii) If the proton signals relax back to equilibrium with two different time constants this would be a hallmark of ortho–para conversion. (iv) Long-lived water can be revealed indirectly by chemical addition onto a suitable substrate such as an activated aldehyde or ketone, monitored by 1H or 13C NMR. For this strategy to be successful, the reactivity of water molecules has to fulfill three requirements that are partly contradictory: (a) the water must be sufficiently diluted so that intermolecular 1H exchange is slowed down; (b) the rate of the chemical addition that is used to reveal the presence of para-water must be faster than the relaxation of the TSI; and (c) the two protons that are added onto the substrate must stem from the same water molecule. Our kinetic measurements show that, under suitable conditions, these requirements may indeed be satisfied.
Results and discussion
Step 1 – preparing a TSI
Unlike Jeener and coworkers19,20 who postulated that the two protons of a H2O molecule can have different environments because of intermolecular dipole–dipole interactions in a magnetic field of 0.7 T, we shall assume that the proton chemical shift anisotropy (CSA) makes significant contributions to the breaking of the symmetry at 6.7 T. We diluted water in deuterated dimethylsulfoxide (DMSO-d6), doped it with ca. 50 mM TEMPOL radicals and froze it to form a glassy state at ca. 1.2 K and 6.7 T. In Fig. 2 we can see proton spectra before (A) and after (B) saturation of the ESR transitions by microwave irradiation at 187.9 GHz with frequency modulation.50 Simulations of H2O powder spectra have been carried out by using the SIMPSON program48 on a spin system made up of two protons with shielding anisotropy ΔCS = −16.19 ppm, asymmetry ηCS = 0.17 and a dipolar coupling d = −30.314 kHz. 4180 crystallite orientations were considered. The relative orientations of the relevant shielding tensors, expressed in a common crystal frame, were given by the Euler angles ΩH(1) = {−165.11°, 115.33°, 36.75°} and ΩH(2) = {−14.89°, 115.33°, −36.75°}, with the dipolar coupling tensor between the two protons oriented according to ΩH(1,2) = {180.00°, 58.83°, 0.00°}. These parameters have been calculated in previous work on water molecules in barium chlorate monohydrate35 by means of DFT and planewave-pseudopotential methods as implemented in the CASTEP code.51 The shielding ellipsoids have their main components aligned along the OH bonds. A realistic pulse was used, with an rf-field strength ν1 = 50 kHz and nutation angles β = 90° and 1.8°, in Fig. 2C and D, respectively. The initial density operator was either ρ(0) = IZ + SZ to describe the high-temperature approximation (Fig. 2C) or ρ(0) = (1/2)[1/2E + IZ + SZ + 2IZSZ] which corresponds to a pure state where only the lowest-lying state is populated at very low spin temperatures (Fig. 2D). The experimental spectra in Fig. 2A and B have larger linewidths than the simulated spectra in Fig. 2C and D: this can be ascribed to the presence of paramagnetic species in solution (TEMPOL) and to intermolecular dipolar couplings that were not considered in the simulations. Finally this evidence suggests that DNP indeed allows one to drive the populations towards the lowest-lying state: since there is some mixing between the central triplet state and the singlet state, a very low spin temperature (on the order of 10 mK in our experiments) is sufficient to generate a TSI. | ||
| Fig. 2 Experimental proton spectra of H2O diluted in DMSO doped with 50 mM TEMPOL and frozen at ca. 1.2 K in a field of 6.7 T, with (A) and without (B) irradiation with a resonant microwave field at 187.9 GHz. Simulations by using SIMPSON48 (see text for details) for initial density operators ρ = IZ + SZ (C) or ρ = (1/2)[1/2E + IZ + SZ + 2IZSZ] (D). The experimental spectra A and B have larger linewidths than the simulated C and D because of intermolecular dipolar couplings and paramagnetic species in the frozen glass. | ||
Step 2 – protecting the TSI during transfer
Proton exchange in water could represent the major source of losses of TSI in our experiment. Hence, we studied the exchange in water in bulk and dilute solutions. The combined effects of proton exchange and proton T1 relaxation on the transverse T2 relaxation of 17O nuclei in H217O can be characterized by using multiple refocusing of transverse 17O magnetization in the manner of Carr, Purcell, Meiboom and Gill (CPMG). One may compare 17O echo decays in the presence or absence of proton decoupling, in analogy with a similar work carried out on 15N.45,46 Our observations shown in Fig. 3 are in agreement with pioneering studies by Meiboom.44 We measured lifetimes τEX = 1/kEX of a few milliseconds in pure water at pH 5.93 and different temperatures. The lifetime of pure water as a molecular entity is clearly too short for our purposes but, as mentioned above, dilution in aprotic solvents can be used to extend this lifetime. In dilute solutions, the proton spectra of mixtures of H2O and HDO feature two distinct resonances. In order to observe HDO triplets due to 1JHD(1H,17O) = 80 Hz, the lifetime of HDO has to be τEX > 1/1JHD(1H,17O) = 12.5 ms. Slow exchange rates can be quantified using selective 1D or 2D exchange spectroscopy (EXSY).47 The pulse sequence used is shown in Fig. 5. Our samples consisted of a mixture of H2O, HDO and D2O diluted in various organic solvents at different concentrations (see Table 1 and Fig. 4). The concentrations [H2O] and [HDO] have been determined by NMR within ±10%, by scaling their peak intensities to an external reference with a concentration that is known a priori. In nitromethane and dioxane, [H2O] and [HDO] were increased with respect to other solvents since, at low concentrations, the proton exchange was too slow to be monitored via 1D-EXSY.| Solvent | Experiments@800 MHz | Fitting@800 MHz | τ EX (s) normalized to [H2O] + [HDO] = 50 mM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| α | [H2O] (mM) | ν(H2O) (ppm) | [HDO] (mM) | ν(HDO) (ppm) | α | R eff (s) | k EX (s−1) | ||
| Dioxane-d8 | 0.28 | 105 | ∼2.71 | 169 | ∼2.67 | 0.30 | 0.19 | 0.14 ± 0.03 | 20 |
| Nitromethane-d3 | 0.26 | 36 | ∼2.13 | 50 | ∼2.10 | 0.40 | 0.08 | 0.30 ± 0.03 | 3 |
| Acetonitrile-d3 | 0.19 | 5 | ∼2.18 | 4 | ∼2.15 | 0.35 | 0.15 | 0.23 ± 0.02 | 0.4 |
| DMSO-d6 | 0.06 | 40 | ∼3.36 | 11 | ∼3.33 | 0.10 | 0.55 | 1.7 ± 0.2 | 0.06 |
| Acetone-d6 | 0.22 | 12 | ∼2.88 | 14 | ∼2.85 | 0.30 | 0.17 | 4.0 ± 0.5 | 0.04 |
| Dichloromethane-d2 | 0.73 | 2 | ∼1.63 | 21 | ∼1.60 | 0.77 | 0.15 | 4.6 ± 0.4 | 0.001 |
 | ||
| Fig. 3 Measurement of fast proton exchange rates kEX in pure water. Proton exchange rates as a function of temperature at pH = 5.93 were extracted from ratios of peak heights of oxygen-17 echoes observed without and with proton decoupling.45,46 The lifetimes τEX = 1/kEX range from 3.6 to 0.9 ms between 275 and 310 K. | ||
 | ||
| Fig. 4 Proton spectrum of 36 mM H2O (singlet on left-hand side) and 50 mM HDO (triplet due to J(H,D) on the right-hand side) in nitromethane-d3 at 300 K and 800 MHz. | ||
 | ||
| Fig. 5 [top] Pulse sequence for selective 1D-EXSY experiments. [bottom] Typical build-up and decay curves measured using 1D-EXSY experiments used for the measurement of the lifetime of water as a molecular entity, when diluted in aprotic solvents (acetonitrile-d3 in this example). Red squares and orange triangles represent intensities of diagonal peaks of H2O and HDO, respectively; green circles describe magnetization transfer from HDO to H2O, while blue rhombi describe the reverse reaction. The rates measured for different solvents are reported in Table 1. | ||
The build-up and decay curves shown in Fig. 5 can be fitted to the following functions (see the Appendix for details):
The parameters α, Reff and kEX have been determined by global fitting to the curves in Fig. 5 and are shown in Table 1. The rates R1 = 1/T1 of H2O have been determined experimentally by inversion recovery (see Table 2). Reff is an average of R1(H2O) and R1(HDO) weighted by their concentrations. Hence, a comparison between Reff in Table 1 and R1 in Table 2 can only be qualitative because of different experimental conditions. However, in most cases the values are similar. In order to facilitate comparisons, in Table 1 we estimated the lifetimes for a concentration [H2O] + [HDO] = 50 mM, assuming that the rates vary linearly with concentration.49 It is evident that, at concentrations below 50 mM, dioxane allows one to extend the lifetime of water as a molecular entity up to a few minutes. Dioxane, therefore, seems a good solvent for dissolution DNP. However, it has a much higher viscosity and lower heat capacity than water, so that our dissolution apparatus has to be re-designed thoroughly.
Step 3 – detecting TSI relaxation
To observe a TSI in H2O at room temperature after dissolution, we have resorted to a chemical reaction, inspired by the PASADENA1 and ALTADENA52 methods. We have shown recently32 that one can lift the degeneracy of the two protons in fumarate (−OOCCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOO−) by addition of D2O to produce malate (−OOCCHDCHODCOO−), a reaction that is catalyzed by fumarase. In our work on para-ethanol,31 instead, the detection was possible, without any chemical reactions, by monitoring non-binomial multiplets in an AX2 system. Following a suggestion by Jean-Maurice Mallet we have explored the addition of water (though not yet of para-water) on aldehydes, i.e., RCHO + H2O → RCH(OH)2. The C
CHCOO−) by addition of D2O to produce malate (−OOCCHDCHODCOO−), a reaction that is catalyzed by fumarase. In our work on para-ethanol,31 instead, the detection was possible, without any chemical reactions, by monitoring non-binomial multiplets in an AX2 system. Following a suggestion by Jean-Maurice Mallet we have explored the addition of water (though not yet of para-water) on aldehydes, i.e., RCHO + H2O → RCH(OH)2. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond of the aldehyde can be activated by substituents such as R = CCl3, as in chloral (CCl3CHO, see Fig. 6). When the reaction is carried out in a dilute solution in acetonitrile, the two water protons (highlighted by stars in Fig. 6) may be assumed to end up on the same hydrate molecule. The two OH protons that stem from para-water give rise to degenerate doublets in the vicinity of 6.4 ppm. The lone proton near 5.3 ppm that stems from the aldehyde gives rise to a triplet due to two equal long-range couplings 3JHH ≈ 6 Hz to the two OH protons. Clearly, as discussed above, deviations from the binomial 1
O double bond of the aldehyde can be activated by substituents such as R = CCl3, as in chloral (CCl3CHO, see Fig. 6). When the reaction is carried out in a dilute solution in acetonitrile, the two water protons (highlighted by stars in Fig. 6) may be assumed to end up on the same hydrate molecule. The two OH protons that stem from para-water give rise to degenerate doublets in the vicinity of 6.4 ppm. The lone proton near 5.3 ppm that stems from the aldehyde gives rise to a triplet due to two equal long-range couplings 3JHH ≈ 6 Hz to the two OH protons. Clearly, as discussed above, deviations from the binomial 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 distribution of this triplet can be used for a quantitative determination of the TSI.31
1 distribution of this triplet can be used for a quantitative determination of the TSI.31
The relative intensities of the lines in these multiplets can give a measure of the TSI in H2O, i.e., of the relative populations of ortho- and para-water, much as in the 17O spectrum of H217O, or in the 13C spectrum of the 13CH2 group in partially deuterated ethanol CD313CH2OD. We therefore explored the possibility of using reactions like in Fig. 6 to observe the binomial distribution of the triplet of the CH2 protons. We explored the kinetics of the addition of H2O onto two different substrates: chloral diluted in acetonitrile and 1,3-dichlorotetrafluoroacetone diluted in dioxane. The main requirement is that the reaction must be faster than the relaxation of the TSI and its decay due to proton exchange. The rate constants found using pseudo-first-order kinetic equations are reported in Table 3, albeit without DNP and thus without TSI. In acetonitrile-d3, with a 5-fold excess of chloral with respect to H2O, a pseudo first-order rate constant kpfo = 0.002 s−1 was observed. This reaction is too slow to be useful for detecting the TSI characteristic of para-water. However, the addition of water onto the more reactive compound (1,3-dichlorotetrafluoroacetone, in dioxane-d8) was so fast that an accurate determination of the rate was difficult by NMR. A reaction rate kpfo > 0.1 s−1 makes it a good candidate as a “revealing agent” of TSI in para-water. As an alternative method for detection, one can simply measure the 17O spectrum in 0.037% natural abundance or with partial isotopic enrichment to improve sensitivity. The 17O spectrum in Fig. 7 shows a triplet due to 1J(1H,17O) = 80 Hz of 55 mM H2O in dioxane-d8 at 298 K and 400 MHz. Using water enriched to 20% 17O, we were able to acquire an 17O spectrum in a few seconds, making this method a valid alternative to the use of a chemical reaction. Again, deviations from the binomial distribution of the intensities of the spectral lines should provide the information needed to assess the lifetime of the TSI and hence of para-water.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds observed at 800 MHz and 300 K
O bonds observed at 800 MHz and 300 K
| Reactant | Solvent | [Reactant] (mM) | [H2O] (mM) | k pfo (s−1) |
|---|---|---|---|---|
| Chloral | Acetonitrile-d3 | 1000 | 200 | 0.002 |
| 1,3-Dichlorotetra-fluoroacetone | Dioxane-d8 | 275 | 55 | >0.1 |
We have optimized the most critical aspects of the experimental scheme suggested in this paper. However, we were not yet able to perform complete experiments since our setup needs to be significantly adapted in order to support dissolution with dioxane.
Conclusions
We have proposed an experimental strategy to produce para-water on a macroscopic scale (i.e. with concentrations in the mM range). Provided that the CSA of the protons is sufficient to lift the degeneracy of the two spins in a frozen sample, we demonstrated that one can use DNP to enhance the population of the ground state of water molecules, thus generating a Triplet–Singlet Imbalance (TSI) that is expected to be a long-lived state analogous to para-water. We proved that the lifetime of water as a molecular entity can be extended up to a few minutes by dilution in aprotic solvents. Several detection strategies can be used either by performing a “revealing” reaction such as the addition of water to an aldehyde or another suitable substrate or by observing the 17O NMR spectrum of water itself. It is also possible to monitor proton magnetization of water relaxing to equilibrium with two clearly distinct time constants to assess the lifetime of the TSI and hence the amount of para-water in the sample. Similar information can, in principle, be obtained by infrared spectroscopy. The detection of the para-water signal with long lifetimes may open the way to study slow transport phenomena such as flow, diffusion, and electrophoretic mobility.Appendix
Let us consider a solution of pure H2O. Let p be the frequency at which a proton is exchanged with another proton belonging to a different water molecule:| p ∝ [H2O] |
Hence, the proton exchange rate kEX can be defined as:
| kEX = 2p |
The factor 2 reflects the fact that a proton belonging to a H2O molecule can exchange with either of the two protons of another H2O molecule.
Let us now consider a mixture of H2O, HDO and D2O. With the ratio α = [H]/([H] + [D]), their concentrations can be expressed as:
| [H2O] = α2CTOT |
| [D2O] = (1 − α)2CTOT |
| [HDO] = 2α(1 − α)CTOT |
| CTOT = [H2O] + [HDO] + [D2O] |
The equations for the exchange of proton magnetization between two distinct sites can be written in the general form:
Let us consider the following reactions involving the exchange of a proton:
| H2O + D2O ↔ 2HDO |
| HDO + D2O ↔ D2O + HDO |
| H2O + HDO ↔ HDO + H2O |
If we focus attention on the magnetization transfer from H2O to HDO, there are three possibilities:
(1) H2O meets another H2O. The exchange of two protons does not lead to any transfer of magnetization between different environments with distinct chemical shifts.
(2) H2O meets D2O. There are four possible exchange processes. Each process leads to the creation of two HDO molecules, and there are two protons that transfer their magnetization between different environments:
| k2 = 8p[D2O] = 8pα2 |
(3) H2O meets HDO. Again, four possible exchange processes can take place, but there is only one proton that transfers its magnetization between different environments:
| k3 = 4p[HDO] = 8pα(1 − α) |
If we consider that we should count an exchange process not only for the proton that hops but also for its neighbor, the total exchange rate is:
If we now consider magnetization transfer from HDO to H2O, there are again three possibilities:
(4) HDO meets H2O. This is symmetric to case (3) above:
| k1 = 4p[H2O] = 4p(1 − α)2 |
(5) HDO meets D2O. Four possible exchange processes can again occur but none of them leads to any magnetization transfer between different environments.
(6) HDO meets HDO. Again, four possible exchange processes are possible, two of which swap a proton with another proton but do not lead to any magnetization transfer. Two processes swap a proton and a deuteron to create two H2O molecules, which is accompanied by a transfer of magnetization between different environments:
| k3 = 4p[HDO] = 8pα(1 − α) |
In this case we have counted the exchange processes twice: when a molecule i meets a molecule j the effect is of course the same as the case when j meets i. Hence the total rate is:
By including longitudinal relaxation, we find:
This matrix can be diagonalized as:
With the assumption that  , we finally find:
, we finally find:
In these calculations we have neglected possible kinetic isotope effects on the rates.
Acknowledgements
Geoffrey Bodenhausen is indebted to at least 16 reviewers who wrote constructive critiques of no less than three proposals submitted to the ERC. The authors thank Martial Rey for valuable assistance and André Merbach, Lothar Helm, Malcolm Levitt, Giuseppe Pileio, Jean-Maurice Mallet, and Jean-Nicolas Dumez for many useful discussions.Notes and references
- C. R. Bowers and D. P. Weitekamp, Phys. Rev. Lett., 1986, 57, 2645–2648 CrossRef CAS.
- C. R. Bowers and D. P. Weitekamp, J. Am. Chem. Soc., 1987, 109, 5541–5542 CrossRef CAS.
- B. Meier, J.-N. Dumez, G. Stevanato, J. T. Hill-Cousins, S. S. Roy, P. Hakansson, S. Mamone, R. C. D. Brown, G. Pileio and M. H. Levitt, J. Am. Chem. Soc., 2013, 135, 18746–18749 CrossRef CAS PubMed.
- J. Haupt, Phys. Lett. A, 1972, 38, 389–390 CrossRef CAS.
- M. Icker, P. Fricke and S. Berger, J. Magn. Reson., 2012, 223, 148–150 CrossRef CAS PubMed.
- M. Tomaselli, C. Degen and B. H. Meier, J. Chem. Phys., 2003, 118, 8559–8562 CrossRef CAS PubMed.
- M. Tomaselli, U. Meier and B. H. Meier, J. Chem. Phys., 2004, 120, 4051–4054 CrossRef CAS PubMed.
- J.-N. Dumez, P. Håkansson, S. Mamone, B. Meier, G. Stevanato, J. T. Hill-Cousins, S. S. Roy, R. C. D. Brown, G. Pileio and M. H. Levitt, J. Chem. Phys., 2015, 142, 044506 CrossRef PubMed.
- M. Carravetta, O. G. Johannessen and M. H. Levitt, Phys. Rev. Lett., 2004, 92, 153003 CrossRef.
- R. Sarkar, P. R. Vasos and G. Bodenhausen, J. Am. Chem. Soc., 2007, 129, 328–334 CrossRef CAS PubMed.
- M. C. D. Tayler and M. H. Levitt, Phys. Chem. Chem. Phys., 2011, 13, 5556 RSC.
- M. C. D. Tayler, I. Marco-Rius, M. I. Kettunen, K. M. Brindle, M. H. Levitt and G. Pileio, J. Am. Chem. Soc., 2012, 134, 7668–7671 CrossRef CAS PubMed.
- G. Stevanato, J. T. Hill-Cousins, P. Håkansson, S. S. Roy, L. J. Brown, R. C. D. Brown, G. Pileio and M. H. Levitt, Angew. Chem., Int. Ed., 2015, 54, 3740–3743 CrossRef CAS PubMed.
- R. Buratto, D. Mammoli, E. Chiarparin, G. Williams and G. Bodenhausen, Angew. Chem., Int. Ed., 2014, 53, 11376–11380 CrossRef CAS PubMed.
- R. Buratto, A. Bornet, J. Milani, D. Mammoli, B. Vuichoud, N. Salvi, M. Singh, A. Laguerre, S. Passemard, S. Gerber-Lemaire, S. Jannin and G. Bodenhausen, ChemMedChem, 2014, 9, 2509–2515 CrossRef CAS PubMed.
- N. Salvi, R. Buratto, A. Bornet, S. Ulzega, I. Rentero Rebollo, A. Angelini, C. Heinis and G. Bodenhausen, J. Am. Chem. Soc., 2012, 134, 11076–11079 CrossRef CAS PubMed.
- R. F. Curl, J. Chem. Phys., 1967, 46, 3220 CrossRef CAS PubMed.
- G. E. Pake, J. Chem. Phys., 1948, 16, 327 CrossRef CAS PubMed.
- H. Eisendrath and J. Jeener, Phys. Rev. B: Solid State, 1978, 17, 54–60 CrossRef CAS.
- H. Eisendrath, W. Stone and J. Jeener, Phys. Rev. B: Solid State, 1978, 17, 47–53 CrossRef CAS.
- T. Kravchuk, M. Reznikov, P. Tichonov, N. Avidor, Y. Meir, A. Bekkerman and G. Alexandrowicz, Science, 2011, 331, 319–321 CrossRef CAS PubMed.
- D. A. Horke, Y.-P. Chang, K. Długołęcki and J. Küpper, Angew. Chem., Int. Ed., 2014, 53, 11965–11968 CrossRef CAS PubMed.
- V. I. Tikhonov, Science, 2002, 296, 2363 CrossRef CAS PubMed.
- S. L. Veber, E. G. Bagryanskaya and P. L. Chapovsky, J. Exp. Theor. Phys., 2006, 102, 76–83 CrossRef CAS.
- M. E. Fajardo, S. Tam and M. E. DeRose, J. Mol. Struct., 2004, 695–696, 111–127 CrossRef CAS PubMed.
- R. L. Redington and D. E. Milligan, J. Chem. Phys., 1962, 37, 2162 CrossRef CAS PubMed.
- R. Sliter, M. Gish and A. F. Vilesov, J. Phys. Chem. A, 2011, 115, 9682–9688 CrossRef CAS PubMed.
- K. Kurotobi and Y. Murata, Science, 2011, 333, 613–616 CrossRef CAS PubMed.
- C. Beduz, M. Carravetta, J. Y.-C. Chen, M. Concistrè, M. Denning, M. Frunzi, A. J. Horsewill, O. G. Johannessen, R. Lawler, X. Lei, M. H. Levitt, Y. Li, S. Mamone, Y. Murata, U. Nagel, T. Nishida, J. Ollivier, S. Rols, T. Room, R. Sarkar, N. J. Turro and Y. Yang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 12894–12898 CrossRef CAS PubMed.
- S. Mamone, M. Concistrè, E. Carignani, B. Meier, A. Krachmalnicoff, O. G. Johannessen, X. Lei, Y. Li, M. Denning, M. Carravetta, K. Goh, A. J. Horsewill, R. J. Whitby and M. H. Levitt, J. Chem. Phys., 2014, 140, 194306 CrossRef PubMed.
- D. Mammoli, B. Vuichoud, A. Bornet, J. Milani, J.-N. Dumez, S. Jannin and G. Bodenhausen, J. Phys. Chem. B, 2015, 119, 4048–4052 CrossRef CAS PubMed.
- A. Bornet, X. Ji, D. Mammoli, B. Vuichoud, J. Milani, G. Bodenhausen and S. Jannin, Chem. – Eur. J., 2014, 20, 17113–17118 CrossRef CAS PubMed.
- K. Modig and B. Halle, J. Am. Chem. Soc., 2002, 124, 12031–12041 CrossRef CAS PubMed.
- P. Tekely, P. Palmas and P. Mutzenhardt, J. Magn. Reson., 1997, 127, 238–240 CrossRef CAS PubMed.
- D. Carnevale, S. E. Ashbrook and G. Bodenhausen, RSC Adv., 2014, 4, 56248–56258 RSC.
- J. Tennyson, J. Phys. Chem. Ref. Data, 2001, 30, 735 CrossRef CAS PubMed.
- J. Milani, B. Vuichoud, A. Bornet, P. Miéville, R. Mottier, S. Jannin and G. Bodenhausen, Rev. Sci. Instrum., 2015, 86, 024101 CrossRef PubMed.
- P. Miéville, P. Ahuja, R. Sarkar, S. Jannin, P. R. Vasos, S. Gerber-Lemaire, M. Mishkovsky, A. Comment, R. Gruetter, O. Ouari, P. Tordo and G. Bodenhausen, Angew. Chem., Int. Ed., 2010, 49, 6182–6185 CrossRef PubMed.
- D. Gajan, A. Bornet, B. Vuichoud, J. Milani, R. Melzi, H. A. van Kalkeren, L. Veyre, C. Thieuleux, M. P. Conley, W. R. Gruning, M. Schwarzwalder, A. Lesage, C. Coperet, G. Bodenhausen, L. Emsley and S. Jannin, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 14693–14697 CrossRef CAS PubMed.
- P. L. Chapovsky, J. Cosléou, F. Herlemont, M. Khelkhal and J. Legrand, Chem. Phys. Lett., 2000, 322, 424–428 CrossRef CAS.
- P. L. Chapovsky and L. J. F. Hermans, Annu. Rev. Phys. Chem., 1999, 50, 315–345 CrossRef CAS PubMed.
- P. Cacciani, J. Cosléou and M. Khelkhal, Phys. Rev. A: At., Mol., Opt. Phys., 2012, 85, 012521 CrossRef.
- N. D. Sergeyeva, V. N. Torocheshnikov and N. M. Sergeyev, Moscow Univ. Chem. Bull., 2010, 65, 98–102 CrossRef.
- S. Meiboom, J. Chem. Phys., 1961, 34, 375 CrossRef CAS PubMed.
- T. Segawa, F. Kateb, L. Duma, G. Bodenhausen and P. Pelupessy, ChemBioChem, 2008, 9, 537–542 CrossRef CAS PubMed.
- F. Kateb, P. Pelupessy and G. Bodenhausen, J. Magn. Reson., 2007, 184, 108–113 CrossRef CAS PubMed.
- J. Jeener, B. H. Meier, P. Bachmann and R. R. Ernst, J. Chem. Phys., 1979, 71, 4546 CrossRef CAS PubMed.
- M. Bak, J. T. Rasmussen and N. C. Nielsen, J. Magn. Reson., 2000, 147, 296–330 CrossRef CAS PubMed.
- N. M. Sergeyev and N. D. Sergeyeva, Phys. Chem. Chem. Phys., 2002, 4, 2994–2999 RSC.
- A. Bornet, J. Milani, B. Vuichoud, A. J. Perez Linde, G. Bodenhausen and S. Jannin, Chem. Phys. Lett., 2014, 602, 63–67 CrossRef CAS PubMed.
- M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717–2744 CrossRef CAS.
- M. G. Pravica and D. P. Weitekamp, Chem. Phys. Lett., 1988, 145, 255–258 CrossRef CAS.
| This journal is © the Owner Societies 2015 |