Open Access Article
Open Access ArticleMolecularly linked 3D plasmonic nanoparticle core/satellite assemblies: SERS nanotags with single-particle Raman sensitivity†
Max
Schütz
and
Sebastian
Schlücker
*
University of Duisburg-Essen, Faculty of Chemistry, Center for Nanointegration Duisburg-Essen (CENIDE), Universitätsstr. 5, 45141 Essen, Germany. E-mail: sebastian.schluecker@uni-due.de; Fax: +49 2011836841; Tel: +49 2011836843
First published on 17th August 2015
Abstract
A fast, generic, and suspension-based route to highly SERS-active assemblies of noble metal nanoparticles (Au, Ag) with small core–satellite gaps and single-particle Raman sensitivity is presented. Rationally designed, heterobifunctional Raman reporters serve as molecular linkers for electrostatic conjugation of the small satellites to the large core.
1. Introduction
Noble metal colloids support localized surface plasmon resonances (LSPR) upon resonant excitation with light.1–3 Very high local electromagnetic field enhancements (“hot spots”) are observed at sharp tips4 and at junctions between two nanoparticles (NPs).5–8 Surface-enhanced Raman scattering (SERS)9,10 from molecules located in the hot spot can be employed for optically probing the corresponding high local field enhancements.11,12The rational design of plasmonic NP assemblies with defined interparticle junctions and high, reproducible SERS signals has recently gathered large interest.13–15 The assembly process can be mediated and efficiently controlled by molecular linkers.16 Examples are biomacromolecules with recognition elements such as DNA17,18 as well as molecules comprising two terminal surface-seeking groups such as dithiols19 or diamines.20
Nanodiagnostic applications of highly SERS-active NPs for the selective detection and localization of analytes exploit the characteristic vibrational Raman signature of reporter molecules on the metal surface.21–23 The nanofabrication of highly SERS-active NP assemblies requires molecular linkers with large Raman scattering cross sections in the core–satellite gap. Ideally, the resulting molecularly linked 3D assemblies exhibit single-particle (single-assembly) SERS sensitivity as demonstrated by correlative SEM/SERS experiments.
Our initial approach towards 3D assemblies of AuNPs with single-particle SERS sensitivity was based on electrostatic binding of negatively charged, citrate-stabilized satellite NPs onto a positively charged core NP coated with an amino-functionalized thin silica shell.13 A central disadvantage of this approach is the relatively large core–satellite gap (>2 nm) due to the silica shell, which reduces the plasmonic enhancement, and also the requirement of several surface modification steps. Here, we present a simple yet generic route to core/satellite assemblies with a small gap (estimated 1–2 nm) using rationally designed heterobifunctional Raman reporters as molecular linkers.
2. Results and discussion
2.1. Synthesis
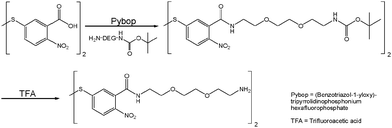
Specifically, we employ 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) as the actual Raman reporter and conjugate it to a bisamino-diethylene glycol (H2N–DEG-NH2) for both hydrophilic stabilization (ethylene glycol)24,25 and subsequent electrostatic conjugation (amino group).26,27 Upon incubation with 80 nm, PVP-capped AuNP cores, the conjugate (DTNB–DEG-NH2) forms a Raman-active (DTNB), positively charged (terminal –NH3+) and hydrophilically stabilized (DEG) self-assembled monolayer (SAM), which replaces PVP by the formation of Au–S bonds28 (Fig. 1 top). Electrostatic binding of negatively charged, citrate-stabilized AuNP satellites (zeta potential up to −30 mV) onto the positively charged core (zeta potential up to +20 mV) leads to 3D core/satellite assemblies,27 which are subsequently encapsulated by a silica shell (Fig. 1 bottom) for chemical and mechanical stabilization, using carboxyethyl silanetriol and tetraethylorthosilicate (TEOS) in a modified Stöber synthesis.29,30Monomeric noble metal nanoparticles were synthesized according to existing protocols. Briefly, small AuNPs for use as satellites were synthesized by reduction of tetrachloroauric(III) acid with sodium citrate. These particles were also used as seeds to grow bigger AuNPs with ∼80 nm in diameter by reduction with hydroquinone for use as core particles.31 Monodisperse Ag cores and satellites were synthesized by reduction of silver nitrate in water/glycerol.32 More details on all syntheses can be found in the section on Materials and methods.
In order to saturate the entire surface of each core with satellites, it is important to employ a very low concentration of core particles in the presence of a large excess of satellites. Due to electrostatic repulsion between the resulting 3D core/satellite superstructures, uncontrolled aggregation does not occur.
The core–satellite gap distance is estimated to be 1–2 nm, based on the length of the heterobifunctional Raman reporter and linker molecule DTNB–DEG-NH2 on the core as well as the thickness of the citrate shell on the satellites. The length of DTNB–DEG-NH2 is a conservative estimation and therefore an upper limit since it is based on an all-trans conformation of the conformationally flexible DEG-NH2 subunits attached to the planar phenyl rings. A precise experimental demonstration of the gap size requires high-resolution TEM, which was not accessible to us when the characterization of the particles was performed. Overall, this approach requires no additional (thicker) silica shell for the encapsulation of the core as in our earlier work13
It is very important to use PVP-capped core particles and citrate-stabilized satellites. Using citrate stabilized cores results in an immediate uncontrolled aggregation upon addition of DTNB–DEG-NH2. On the other hand, PVP-capped satellites are not as good for forming assemblies as citrate-capped satellites are. These observations can be explained by the surface affinity of the bifunctional crosslinker. In the case of PVP-capped core particles, the crosslinker binds via the thiol group to the gold surface of the core particles. The terminal amino groups of the resulting positively charged cores (NH3+) have a high affinity to the negatively charged, citrate-stabilized satellite particles. In contrast, the affinity to PVP-capped satellites is significantly lower. The uncontrolled aggregation observed upon the addition of DTNB–DEG-NH2 to citrate-capped core particles is attributed to charge neutralization and/or binding between two cores at once.
The presented approach is not limited to AuNP cores and AuNP satellites. We synthesized various 3D noble metal NP assemblies using either AgNPs or AuNPs as core particles in combination with smaller AgNPs or AuNPs as satellites. The ethylene glycol units provide stability in aqueous suspension and generate a robust SAM-coated core particle.24,25 Important is only the capping agent: PVP for the core particles and citrate for the satellites. Four combinations are possible: gold core and silver satellites (Ag@Au), gold core and satellites (Au@Au), silver core and satellites (Ag@Ag) as well as gold core and silver satellites (Ag@Au).
2.2. Characterization
We obtained all four types of assemblies and characterized them by TEM. Fig. 2 displays TEM images of different noble metal NP assemblies prior to (A–D) and after silica coating (E and F). The obtained particles are relatively homogeneous and the number of satellites on each core particle is more or less similar. Fig. 3 contains more TEM images (bottom) and also the UV/Vis extinction spectrum (top) for the Ag@Ag superstructures. TEM images and extinction spectra of the other three types of superstructures can be found in the ESI† (Fig. S1–S4).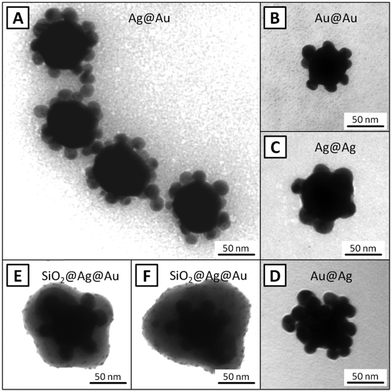 | ||
| Fig. 2 TEM images of different 3D superstructures: Ag@Au (A), Au@Au (B), Ag@Ag (C), Au@Ag (D) and also silica-encapsulated Ag@Au (E and F). See ESI† for more images. | ||
 | ||
| Fig. 3 (top) Normalized UV-VIS extinction spectra of Ag@Ag 3D superstructures (red: directly after the synthesis; black: after centrifugation and redispersion in ethanol) and their precursors (green: Ag satellites; blue: DTNB–DEG-covered core particles). The plasmon band of the 3D superstructures in ethanol is λmax = 588 nm. (bottom) Display TEM images of the Ag@Ag 3D superstructures. See ESI† for more images. | ||
The number of satellites assembled onto each core cannot be quantitatively determined since the TEM images do not give a complete 3D picture of the surface coverage. The plasmon peaks for all four superstructures exhibit maxima between 588 nm (Ag@Ag) and 632 nm (Au@Au), revealing the plasmonic coupling between the core and the satellites. In order to confirm the plasmonic coupling between core and satellites via their anticipated high SERS activity, the 3D plasmonic assemblies were investigated in correlative single-particle SEM/SERS experiments. The results are displayed in Fig. 4. First, SEM images were recorded (Fig. 4A) from assemblies in an area where no other NPs were present. In the next step the same area was investigated by confocal Raman microspectroscopy with 1s integration time and 1 mW laser power. The set of Raman spectra was used to generate a false color image (Fig. 4C) based on the intensity of the dominant Raman band of DTNB at ∼1340 cm−1, as highlighted in the SERS spectrum of the 3D assembly (marked with a cross in Fig. 4C) in Fig. 4B.
Our results show an intense Raman signal at the single-particle level, which is significantly stronger than in our previous work13 and therefore supports the conclusion on the presence of a shorter gap. A direct quantitative comparison, however, is not possible due to differences in the experimental conditions, in particular acquisition times, size of core and satellites as well as the number of satellites.
In summary, a simple, fast, generic and suspension-based synthesis of rationally designed silica-encapsulated and spectrally encoded 3D plasmonic assemblies is presented. The approach is based on a heterobifunctional crosslinker which also serves as the Raman reporter and is present as a SAM on the surface of the core NP (Au or Ag). Upon addition of smaller satellite particles (Au or Ag), the assemblies form immediately due to molecularly assisted self-assembly using Coulomb attraction. In contrast to solid phase-based approaches,27 this suspension-based approach leads to 3D plasmonic superstructures comprising cores located not only on a single hemisphere. In contrast to NP dimers, assemblies of NPs exhibit a quasi-polarization-independent SERS response at the single-particle level.33 In further studies the silica-encapsulated 3D superstructures may be used as SERS labels in bioanalytical and biomedical applications, e.g. in SERS microscopy for tissue-based cancer diagnostics.
3. Materials and methods
Syntheses
1H-NMR (500 MHz, CDCl3) δ [ppm] = 3.16 (m, 2H, CH2); 3.58 (m, 4H, CH2); 3.69 (m, 6H, CH2) 7.71 (s, 1H, arom.); 7.86 (d, 1H, arom.); 8.04 (d, 1H, arom.); 8.74 (s, 1H, NH-CH2). m/z (H+): 657.20 u (calc: 657.2012 u).
The 3D superstructures form immediately by adding for instance 100 μl of the concentrated seed particle suspension to 50 μl of the SAM-functionalized core particles in water. Afterwards the suspension was slowly centrifuged (15 min, 250 g) to avoid unwanted aggregation and the obtained superstructures were redispersed in ethanol for instance. The samples can be washed more often in order to remove all satellite particles. However, this is not mandatory for encapsulation.
Materials
Sigma-Aldrich: 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), dichloromethane (DCM), (benzotriazol-1-yloxy)tripyrrolidi-nophosphonium hexafluorophosphate (Pybop), 4-(dimethyl-amino)pyridine (DMAP), tert-butyl 2-[2-(2-aminoethoxy) ethoxy]ethylcarbamate (DEG-NH2), magnesium sulfate, silver nitrate, tetrachloroauric(III) acid (30%), ethanol, hydroquinone (HQ), 2-propanol, ammonium hydroxide 30%.Applichem: polyvinylpyrrolidone K30 (PVP), glycerol, hydrochloric acid, trifluoroacetic acid (TFA), sodium citrate dehydrate, L-ascorbic acid, methanol, N,N-dimethylformamide (DMF).
ABCR: carboxyethylsilanetriol, sodium salt; 25% in water.
Methods
UV-Vis extinction spectroscopy: Perkin Elmer, Lambda 35. Zetapotential: Malvern Zetasizer Nano Z. Transmission electron microscopy (TEM): Zeiss, EM 902. Scanning electron microscopy (SEM): Zeiss, Supra 50. SERS microscopy: WITec Alpha 300R confocal microscope with a Olympus 40× objective, a HeNe laser (λex = 632.8 nm), a monochromator with 30 cm focal length (600 grooves per mm grating) equipped with an EM-CCD (Andor, Newton).Acknowledgements
Financial support from the European Union and the State of Lower Saxony (EFRE, project no. W2-80111700) is acknowledged. Thanks to Ulrich Krupp for access to FE-SEM and Bernd Walkenfort as well as Dennis Goesling for technical support.Notes and references
- V. Giannini, A. I. Fernández-Domínguez, S. C. Heck and S. A. Maier, Chem. Rev., 2011, 111, 3888 CrossRef CAS PubMed.
- R. Aroca, Surface-Enhanced Vibrational Spectroscopy, Wiley VCH, 2006 Search PubMed.
- E. Ringe, B. Sharma, A.-I. Henry, L. D. Marks and R. P. V. Duyne, Phys. Chem. Chem. Phys., 2013, 15, 4110 RSC.
- C. L. Nehl, H. Liao and J. H. Hafner, Nano Lett., 2006, 6, 683 CrossRef CAS PubMed.
- E. C. L. Ru, P. G. Etchegoin and M. Meyer, J. Chem. Phys., 2006, 125, 204701 CrossRef PubMed.
- R. Jin, Angew. Chem., Int. Ed., 2010, 49, 2826 CrossRef CAS PubMed.
- J. P. Camden, J. A. Dieringer, Y. Wang, D. J. Masiello, L. D. Marks, G. C. Schatz and R. P. Van Duyne, J. Am. Chem. Soc., 2008, 130, 12616 CrossRef CAS PubMed.
- J. M. McMahon, S. Li, L. K. Ausman and G. C. Schatz, J. Phys. Chem. C, 2012, 116, 1627 CAS.
- E. L. Ru and P. Etchegoin, Principles of Surface-Enhanced Raman Spectroscopy and related plasmonic effects, Elsevier, 2008 Search PubMed.
- S. Schlücker, Angew. Chem., Int. Ed., 2014, 53, 4756 CrossRef PubMed.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102 CrossRef CAS PubMed.
- J. A. Dieringer, R. B. Lettan, K. A. Scheidt and R. P. Van Duyne, J. Am. Chem. Soc., 2007, 129, 16249 CrossRef CAS PubMed.
- M. Gellner, D. Steinigeweg, S. Ichilmann, M. Salehi, M. Schütz, K. Kömpe, M. Haase and S. Schlücker, Small, 2011, 7, 3445 CrossRef CAS PubMed.
- D. Craig, J. Simpson, K. Faulds and D. Graham, Chem. Commun., 2013, 49, 30 RSC.
- L. Guerrini, F. McKenzie, A. W. Wark, K. Faulds and D. Graham, Chem. Sci., 2012, 3, 2262 RSC.
- L. Guerrini and D. Graham, Chem. Soc. Rev., 2012, 41, 7085 RSC.
- D.-K. Lim, K.-S. Jeon, H. M. Kim, J.-M. Nam and Y. D. Suh, Nat. Mater., 2010, 9, 60 CrossRef CAS PubMed.
- D. Graham, D. G. Thompson, W. E. Smith and K. Faulds, Nat. Nanotechnol., 2008, 3, 548 CrossRef CAS PubMed.
- A. S. D. S. Indrasekara, B. J. Paladini, D. J. Naczynski, V. Starovoytov, P. V. Moghe and L. Fabris, Adv. Healthcare Mater., 2013, 2, 1370 CrossRef CAS PubMed.
- B. Vlcková, M. Moskovits, I. Pavel, K. Sisková, M. Sládková and M. Slouf, Chem. Phys. Lett., 2008, 455, 131 CrossRef PubMed.
- W. E. Doering and S. Nie, Anal. Chem., 2003, 75, 6171 CrossRef CAS PubMed.
- S. P. Mulvaney, M. D. Musick, C. D. Keating and M. J. Natan, Langmuir, 2003, 19, 4784 CrossRef CAS.
- Y. Wang and S. Schlücker, Analyst, 2013, 138, 2224 RSC.
- C. Jehn, B. Küstner, P. Adam, A. Marx, P. Ströbel, C. Schmuck and S. Schlücker, Phys. Chem. Chem. Phys., 2009, 11, 7499 RSC.
- Y. Wang, M. Salehi, M. Schütz, K. Rudi and S. Schlücker, Analyst, 2013, 138, 1764 RSC.
- J. H. Yoon and S. Yoon, Langmuir, 2013, 29, 14777 CrossRef PubMed.
- J. H. Yoon, Y. Zhou, M. G. Blaber, G. C. Schatz and S. Yoon, J. Phys. Chem. Lett., 2013, 4, 1371 CrossRef CAS PubMed.
- X.-L. Tang, P. Jiang, G.-L. Ge, M. Tsuji, S.-S. Xie and Y.-J. Guo, Langmuir, 2008, 24, 1763–1768 CrossRef CAS PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- C. Graf, D. L. J. Vossen, A. Imhof and A. van Blaaderen, Langmuir, 2003, 19, 6693 CrossRef CAS.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042 CrossRef CAS PubMed.
- D. Steinigeweg and S. Schlücker, Chem. Commun., 2012, 48, 8682 RSC.
- D. Steinigeweg, M. Schütz and S. Schlücker, Nanoscale, 2013, 5, 110 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Further data. See DOI: 10.1039/c5cp03189c |
| This journal is © the Owner Societies 2015 |