Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Energy-dependent gas-phase fragmentation of fluorofullerene multiply charged anions (MCAs)
Rolf W.
Kirschbaum
a,
Markus
Hausmann
a,
Olga V.
Boltalina
b,
Steven H.
Strauss
b and
Thomas
Drewello
*a
aPhysical Chemistry I, Department of Chemistry and Pharmacy, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Egerlandstraße 3, 91058 Erlangen, Germany. E-mail: thomas.drewello@fau.de
bDepartment of Chemistry, Colorado State University, Fort Collins, CO 80523, USA
First published on 6th August 2015
Abstract
Long-lived di- and trianions have been formed from fluorofullerenes in the gas phase by electrospray ionization. Fragmentation of multiply charged anions has been induced by multiple low-energy collisions. Two complementary dissociation experiments have been conducted. Firstly, unequivocal connectivity has been established between the precursor ion and the first and second generation of its product ions. Secondly, dianions have been studied in energy-resolved collisions, allowing the elucidation of the energetic demands of the dissociations. It was possible to study dianions with odd and even F numbers, possessing even and odd electron configurations, respectively. The fragmentation behavior is governed by the electronic stability of the anionic protagonists, in that ions with the less stable odd-electron configuration dissociate into species with the more stable even-electron configuration. Dianions with an odd electron count release an F˙ atom to turn into an even electron system while retaining the charge state. Dianions with an even electron count undergo a more energy demanding charge separation reaction (Coulomb explosion) into an F− anion and an even electron monoanion. The studied trianions behave accordingly. F2 loss is prominent only with monoanions within an even-to-even electron fragmentation cascade. The trianions are long-lived with lifetimes of at least 0.1 s.
Introduction
Multiply charged anions (MCAs) are amongst the most elusive species in the gas phase.1–4 In solution or in the condensed phase MCAs are stabilized by interaction with suitable partners. However, when transferred into the gas phase, their stability entirely depends on the ability to accommodate several repulsive charges on one isolated entity. Some of the most common dianions in solution, such as CO32− or SO42−, could not be generated in the gas phase.1–4 Those MCAs that showed sufficient stability have attracted attention and different approaches have been developed to study MCAs in the gas phase.1–4 Stable gas phase dianions of fluorofullerenes have long been known and have been the topic of several investigations.5–14 Fluorofullerene dianions have been generated in various ways, including double electron attachment,5,14 electrochemical production followed by electrospray ionization-based transfer into the gas phase,13 electrospray ionization (ESI) from solutions doped with organic electron donor compounds,6,8 direct ESI9 and high-kinetic energy collisions, causing electron transfer to a monoanionic projectile fluorofullerene ion from gaseous sodium7 and other gases.10–12 In the latter experiments it has been even possible to generate long-lived fluorofullerene trianions.15 In fluorofullerenes the combination of molecular size, the presence of strong covalent CC and CF bonds, as well as high electronegativity leads to a favorable situation in which the dianions are particularly stable. The fluorofullerene dianions have been found to be even more stable towards electron detachment than the corresponding monoanions.14 Fluorofullerenes possess a large second electron affinity (EA2). In the present context, the EA2 is defined as the energy difference between mono- and dianions and positive values are assigned to thermochemically stable dianions. The EA2 has been determined, for instance, by photoelectron spectroscopy as the adiabatic electron detachment energy of the dianion. For C60F342− formed from C60F36 an EA2(C60F34) = 2.50(5) eV6 [2.4(1) eV]8 and for C60F462− formed from C60F48 an EA2(C60F46) = 3.33(10) eV6 [3.2(1) eV]8 have been determined. Compared with non-fluorinated fullerenes, these values are fairly high. The EA2 of C60 has been established to be even negative16 and the EA2 values of C76, C78 and C84 were measured to lie between 0.3 and 0.8 eV.17,18 Of essential importance in the context of stability of the MCA towards electron detachment is the presence of a repulsive Coulomb barrier (RCB).14,19 The RCB arises as a combination of the binding interactions of the extra electron with the remainder of the MCAs in the short range and the charge repulsion in the long range, resulting in a potential, which may prevent spontaneous electron autodetachment. The even electron trianion C60F473− is among the very few trianions that could be detected at all in the gas phase. It was generated in high-energy collisions of C60F47− with Na as the collision gas by successive double electron attachment. While the third electron affinity of C60F473− was calculated to be negative, leaving the trianion thermochemically unstable, the third electron is supposed to be trapped by a sufficiently high RCB.15In the present investigation, di- and trianions are formed from fluorofullerenes by direct electrospray ionization. These stable and long-lived MCAs are activated by low-energy collisions and the resulting dissociation behavior is recorded in MSn experiments using an ion trap mass spectrometer and establishing a fragmentation pedigree, providing unequivocal connectivity of the precursor and product ions of successive generations. The dianions with odd and even F numbers could be studied in a second collision experiment using a Qq-TOF mass spectrometer, allowing the study of energy-dependent dissociations of the dianionic precursor ions.
Experimental section
Materials and reagents
The fluorofullerene sample was of commercial origin and prepared by fluorination of fullerene with elemental fluorine. Similar samples have been the subject of earlier investigations into laser desorption/ionization and monoanion CID,20 determination of ionization energies by synchrotron radiation21 and ion formation by nanospray ionization.22Sample preparation
Fluorinated fullerenes were dissolved either in acetonitrile (MeCN) or N,N-dimethylformamide (DMF), at a concentration of roughly 10−5 mol L−1. Since fluorofullerenes decompose quickly in polar solvents, all samples were freshly prepared for consistent results. The samples were introduced into the ion source employing a single-syringe infusion pump (Cole Parmer, Vernon Hills, Illinois).MSn experiments
MSn experiments were carried out using an ESI-quadrupole ion trap instrument (esquire6000, Bruker) with the following settings. Sample flow rate, 4.0 μL min−1; nebulizer nitrogen pressure, 689 hPa; capillary entrance voltage, +4000 V; spray shield voltage, +3500 V; nitrogen dry gas temperature, 573 K; dry gas flow rate, 5.0 L min−1; and the helium buffer/collision gas pressure was set to 4.0 × 10−6 hPa (the actual pressure in the analyzer is approximately 100 times higher23). Generally, the ion transfer settings vary due to spectrum tuning.Energy-dependent collision-induced dissociations (CID)
Energy-dependent dissociation experiments were obtained using an electrospray quadrupole/time-of-flight (ESI-Qq-TOF) instrument (micrOTOF-Q II, Bruker). The following settings were applied. Flow rate, 3.0 mL min−1; nebulizer nitrogen pressure, 400 hPa; capillary entrance voltage, +5000 V; spray shield voltage, +4500 V; nitrogen dry gas temperature, 453 K; and dry gas flow rate, 4.0 L min−1. Collision-induced dissociations (MS2-CID) were conducted in the collision cell q located between Q and TOF, following mass selection and preceding the high-resolution daughter ion analysis in the TOF analyzer. Nitrogen (N2) was used as the collision gas. The collision voltage was varied between 5 and 200 V. The precursor ions were selected with an isolation width of m/z 10. The recorded spectra were processed using DataAnalysis 4.0 software (Bruker).Energy-dependent survival yields
In order to compare the stability of the fluorofullerene dianions the survival yield (SY)24–27 of the precursor ion species was plotted as a function of the collision energy in the center-of-mass frame (ECOM), which is expressed by eqn (1) with the laboratory-frame collision energy ELAB, the mass of the collision-gas molecule mcollision gas and the mass of the precursor ion mprecursor ion.24–34 | (1) |
 | (2) |
Results and discussion
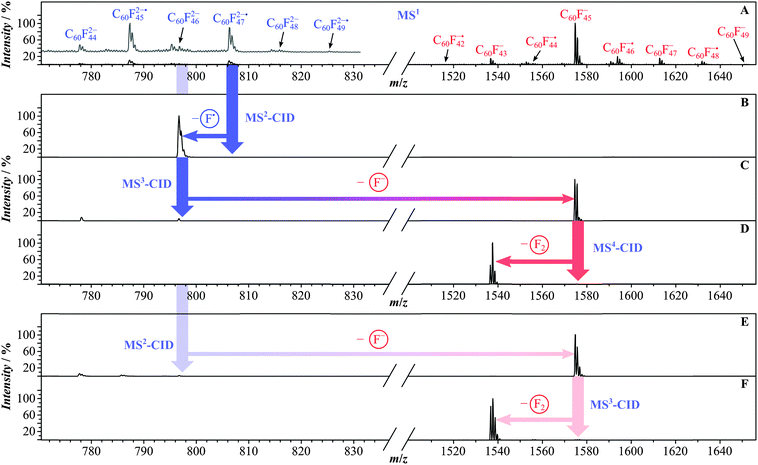
Fig. 1 shows the negative-ion distribution obtained by electrospraying fluorofullerenes from two different solvents. Fig. 1A was obtained using MeCN as the solvent and Fig. 1B using DMF. Evidently, the nature of the solvent has a profound influence on the resulting ion distribution. ESI from MeCN (Fig. 1A) leads to a distribution in which the monoanions are more abundant than the dianions. Within the dianion population, species with an odd F number are more pronounced than the dianions with even F content. When sprayed from DMF, the situation is reversed with the dianions being more abundant than monoanions and dianions with even F content prevailing over those with odd F count. The electrochemical nature inherent to the ESI process plays an essential role in the ion formation under the present conditions.9,22,35,36 However, several processes may influence the final appearance of the spectrum. The initial electrochemical reduction event is not the only relevant process. Decomposition of neutral species as well as dissociation of initially formed ions may contribute as well. It was shown, for instance, that the tailor-made formation of C60F482− dianions by electrochemical reduction followed by ESI-based detection of possible ions would only result in the detection of C60F47− monoanions.13 Equally, further electrochemical reduction of C60F482− into C60F483−˙ would only lead to the detection of C60F472−˙ dianions formed by F− release from the trianion.13 C60F482− underwent complete dissociation into F− and C60F47−. While the underlying processes that lead to the different ion distributions (Fig. 1) remain unresolved, the practical advantage has to be seen in the fact that dianions with both even and odd F content can be formed deliberately by simply changing the solvent. In this way, for the study of odd F content dianions MeCN was used as the solvent and for the study of dianions with even F count DMF was employed.In Fig. 2 the fragmentation behavior is established by MSn experiments, using C60Fn2− dianions with odd (n = 47) and even F content (n = 46) as the initial precursor ions. The fragmentation pattern is straightforward and clean. C60F472−˙ (Fig. 2A) shows the loss of an F˙ atom leading to the C60F462− dianion with even F content (Fig. 2B). Selection and further activation of the latter ion (MS3) lead to a Coulomb explosion into F− (below the detection limit of the ion trap) and C60F45− (Fig. 2C). A reaction that leads to separation of equal charges is here referred to as a Coulomb explosion.37 The alternative reaction path of neutral losses into smaller dianions occurs for the C60F462− dianion only to a very minor extent, producing a minute amount of C60F442− product ion by F2 loss. In the final MS4 experiment (Fig. 2D), the C60F45− monoanion undergoes F2 loss to produce C60F43−. This fragmentation behavior seems to be governed by the tendency to produce product ions of enhanced electronic stability, which are characterized by an even electron count, signifying the closed shell system. For dianions the even electron configuration is reached with an even-numbered F atom content, whereas mono- and trianions would require an odd F number. The starting C60F472−˙ dianion does not undergo Coulomb explosion by F− loss, as the complementary fragment ion C60F46−˙ would be an odd electron ion and as such is less stable. Instead the loss of neutral F˙ leads to the even electron ion C60F462−. All further fragmentations would start from an even-electron ion and lead to even electron fragment ions. In Fig. 2A, E and F, the MSn experiments are shown, starting with the C60F462− dianion generated in the ESI source to allow comparison with the C60F462− dianion produced from C60F472−˙ by CID in the ion trap (Fig. 2A–D). Both dianions show essentially the same dissociation behavior. An earlier odd/even alternation was discovered in dissociative electron capture experiments with fluorinated fullerene cations.11 It was found that the formation of odd F-containing dianions was accompanied by the preferred loss of just one F˙ atom, while even F-containing dianions were preferably accompanied by the loss of two F atoms. Both the present and earlier11 odd/even alternation are driven by the enhanced stability of the even electron system.
In the next set of experiments, the fragmentation behavior of the fluorofullerene dianions is studied in energy-dependent collision-induced dissociations employing a Qq-TOF instrument. In these experiments, the dianion of interest is selected using a quadrupole mass filter and allowed to enter a quadrupole that functions as a collision cell at a well-defined kinetic energy, which is varied stepwise between z × 5 eV and z × 200 eV (z being the charge number of the precursor ion). During the flight through the collision cell quadrupole, the projectile precursor ion experiences multiple low-energy collision events. The internal energy content of the ion increases and fragmentation leads to product ions. The product ions may also be activated and undergo further dissociations. By increasing the kinetic energy of the incoming ion, the extent of fragmentation can be increased and a cascade of successive reactions may be extended. After the collision cell, the ion distribution is recorded using a TOF analyzer. The plot of the relative ion distribution as a function of collision energy is commonly referred to as a breakdown graph. The breakdown graph provides insight into the energetic demands of the dissociation and may also reveal the occurrence of parallel vs. successive reaction pathways.38 We note that CID using collisions in the ion trap would not result in a similar extent of fragmentation as only the parent ions are activated and not the resulting product ions.39Fig. 3A displays the breakdown graphs of the (odd electron) C60F452−˙ dianion. Upon increasing collision energy, F˙ atom loss leads to the formation of the (even electron) C60F442− dianion, which upon further increase of the collision energy undergoes a Coulomb explosion into the (even electron) C60F43− monoanion, which undergoes successive F2 losses into smaller even-electron monoanions. Fig. 3B shows the corresponding breakdown graph of the C60F442− dianion. It is confirmed that the Coulomb explosion of the even electron dianion requires roughly twice (factor 5/3) the collision energy needed for the F˙ atom loss from the less favored odd electron dianion. Moreover, comparing Fig. 3A and B, the almost perfect match of various successive reactions in both figures is apparent. The sequential reactions start in both cases with the C60F442− dianion, in one case it is generated in the ESI source (Fig. 3B) and in the other case by CID from C60F452−˙ (Fig. 3A). Evidently, both processes afford here dianions with very similar internal energy content.
In the following, we discuss the energy-dependent dissociations of different dianionic precursor ions featuring even and odd F content. The decline of the precursor ion abundance is displayed as a function of ECOM (Fig. 4). The collision energy at which the precursor ion has declined to half of its initial abundance is referred to as x50 value (=ECOM,50) and is taken as a measure of the relative stability of the ions in the collision experiment. The dianions C60Fn2−˙ with n = 45, 47 and 49 of odd electron amount are compared to the even electron dianions C60Fn2− with n = 42, 44 and 46. The odd electron dianions show an x50 value of 1.6 eV for the F˙ loss und the even electron dianions display an x50 value of 2.7 eV for the F− loss (Coulomb explosion). It is confirmed that the even electron dianions are more stable, resisting the Coulomb explosion up to an ECOM that is by a factor of 5/3 higher than the ECOM required for the less stable odd electron dianions to undergo F˙ loss. The driving force facilitating the F˙ loss seems to result from the transition of odd to even electron configuration in the dianion.
 | ||
| Fig. 4 Survival yield (SY) vs. collision energy in the center-of-mass frame (ECOM) of even electron (EE) and odd electron (OE) fluorofullerene dianions measured using an ESI-Qq-TOF mass spectrometer. | ||
The final set of experiments is again conducted using the ESI-ion trap instrument, with which the detection of triply charged anions of the form C60Fn3− with n = 43 and 45 was possible. The three extra electrons reside on a fluorofullerene molecule that has undergone the formal loss of one F˙ atom to obtain an odd F number. The resulting trianion is of even electron count and thus the more stable variant to the true molecular trianion, which could not be observed in these experiments. Trianions of observed type have been produced before by high-energy collision-induced double electron attachment from the corresponding monoanion.15 While the third electron affinity has been predicted to be negative, so that the trianion is thermochemically unstable, the existence of a substantial RCB of around 1 eV has been estimated to prevent electron loss from the trianion.15 In the initial experiment, the time between the collision event that formed the trianion and its detection limited its lifetime to a maximum of about 10 μs. In the present study the trianions are formed by direct ESI using an ion trap for detection, which translates into a lifetime of at least 0.1 s, extending the lifetime of the trianions by five orders of magnitude and confirming that these trianions are in fact long-lived.
The fragmentation behavior of the C60F453− trianion can be predicted from the dianion behavior. An odd electron trianion (which was not observed here) would be expected to release an F˙ atom to turn into an even electron trianion. The even electron trianion would be expected to undergo a Coulomb explosion by F− loss to produce the even electron dianion. This is exactly the behavior that has been observed in Fig. 5. The MS2 of C60F453− shows a Coulomb explosion into the F−/C60F442− ion pair and MS3 of the C60F442− dianion shows a second, successive Coulomb explosion into F−/C60F43−. Unfortunately, the trianions could not be generated on the ESI-Qq-TOF instrument to allow energy-resolved dissociations to be conducted.
Conclusions
The fragmentation dynamics of fluorofullerene di- and trianions is governed by their electronic stability. The driving force for the dissociations is the formation of ions with an even electron count and closed shell electron configuration. Uneven electron MCAs show the loss of an F˙ atom to turn into an even electron MCA. Even electron MCAs undergo F− anion loss in a Coulomb explosion, reducing the charge state of the MCA by one. The F˙ loss proceeds from the less stable electron configuration and is less energy demanding than the Coulomb explosion.Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG) – SFB 953 “Synthetic Carbon Allotropes” for financial support.References
- X.-B. Wang and L.-S. Wang, Photoelectron Spectroscopy of Multiply Charged Anions, Annu. Rev. Phys. Chem., 2009, 60, 105–126 CrossRef CAS PubMed.
- W. E. Boxford and C. E. H. Dessent, Probing the Intrinsic Features and Environmental Stabilization of Multiply Charged Anions, Phys. Chem. Chem. Phys., 2006, 8, 5151–5165 RSC.
- S. Feuerbacher and L. S. Cederbaum, Stable and Long-Lived Trianions in the Gas Phase, J. Phys. Chem. A, 2005, 109, 11401–11406 CrossRef CAS PubMed.
- A. Dreuw and L. S. Cederbaum, Multiply Charged Anions in the Gas Phase, Chem. Rev., 2001, 102, 181–200 CrossRef.
- R. F. Tuktarov, R. V. Khatymov, V. Y. Markov, N. A. Romanova and M. V. Muftakhov, Formation of Doubly Charged Negative Ions Under the Conditions of the Resonant Electron Capture by Fluorofullerenes, JETP Lett., 2013, 96, 664–667 CrossRef CAS.
- X.-B. Wang, C. Chi, M. Zhou, I. V. Kuvychko, K. Seppelt, A. A. Popov, S. H. Strauss, O. V. Boltalina and L.-S. Wang, Photoelectron Spectroscopy of C60Fn− and C60Fm2− (n = 17, 33, 35, 43, 45, 47; m = 34, 46) in the Gas Phase and the Generation and Characterization of C1-C60F47− and D2-C60F44 in Solution, J. Phys. Chem. A, 2010, 114, 1756–1765 CrossRef CAS PubMed.
- A. V. Streletskiy, P. Hvelplund, S. B. Nielsen, B. Liu, S. Tomita, A. A. Goryunkov and O. V. Boltalina, Differences in Electronic Properties of Fluorinated and Trifluoromethylated Fullerenes Revealed by Their Propensity for Dianion Formation, J. Chem. Phys., 2006, 124(144306), 1–4 Search PubMed.
- I. N. Ioffe, S. M. Avdoshenko, O. V. Boltalina, L. N. Sidorov, K. Berndt and J. M. Weber, Mass Spectrometry, Photoelectron Spectroscopy, and Quantum Chemical Studies of Fluorofullerene Dianions, Int. J. Mass Spectrom., 2005, 243, 223–230 CrossRef CAS.
- T. Drewello, H. Frauendorf, R. Herzschuh, A. A. Goryunkov, S. H. Strauss and O. V. Boltalina, The Formation of Long-Lived Fluorofullerene Dianions by Direct Electrospray Ionization, Chem. Phys. Lett., 2005, 405, 93–96 CrossRef CAS.
- A. A. Tuinman and R. N. Compton, Doubly Charged Negative Ions via Charge-Exchange Collisions: C60F36− + CH4 → C60F362− + CH4+, Phys. Rev. A: At., Mol., Opt. Phys., 2002, 65(052724), 1–3 Search PubMed.
- O. V. Boltalina, P. Hvelplund, T. J. D. Jørgensen, M. C. Larsen, M. O. Larsson and D. A. Sharoitchenko, Electron Capture by Fluorinated Fullerene Anions in Collisions with Xe Atoms, Phys. Rev. A: At., Mol., Opt. Phys., 2000, 62(023202), 1–7 Search PubMed.
- O. V. Boltalina, P. Hvelplund, M. C. Larsen and M. O. Larsson, Electron Capture by C60F35− in Collisions with Atomic and Molecular Targets, Phys. Rev. Lett., 1998, 80, 5101–5104 CrossRef CAS.
- F. Zhou, G. J. Van Berkel and B. T. Donovan, Electron-Transfer Reactions of C60F48, J. Am. Chem. Soc., 1994, 116, 5485–5486 CrossRef CAS.
- C. Jin, R. L. Hettich, R. N. Compton, A. Tuinman, A. Derecskei-Kovacs, D. S. Marynick and B. I. Dunlap, Attachment of Two Electrons to C60F48: Coulomb Barriers in Doubly Charged Anions, Phys. Rev. Lett., 1994, 73, 2821–2824 CrossRef CAS PubMed.
- O. V. Boltalina, A. V. Streletskii, I. N. Ioffe, P. Hvelplund, B. Liu, S. B. Nielsen and S. Tomita, Formation of Long-Lived Fluorofullerene Trianions in Collisions with Na, J. Chem. Phys., 2005, 122(021102), 1–3 Search PubMed.
- C. Yannouleas and U. Landman, Stabilized-Jellium Description of Neutral and Multiply Charged Fullerenes C60x±, Chem. Phys. Lett., 1994, 217, 175–185 CrossRef CAS.
- O. T. Ehrler, F. Furche, J. M. Weber and M. M. Kappes, Photoelectron Spectroscopy of Fullerene Dianions C762−, C782−, and C842−, J. Chem. Phys., 2005, 122(094321), 1–8 Search PubMed.
- X.-B. Wang, H.-K. Woo, J. Yang, M. M. Kappes and L.-S. Wang, Photoelectron Spectroscopy of Singly and Doubly Charged Higher Fullerenes at Low Temperatures: C76−, C78−, C84− and C762−, C782−, C842−, J. Phys. Chem. C, 2007, 111, 17684–17689 CAS.
- A. Dreuw and L. S. Cederbaum, Nature of the Repulsive Coulomb Barrier in Multiply Charged Negative Ions, Phys. Rev. A: At., Mol., Opt. Phys., 2000, 63(012501), 1–13 Search PubMed.
- L. Käseberg, R. Herzschuh, S. Suslov, O. V. Boltalina, R. Cozzolino, O. Belgacem and T. Drewello, Laser Desorption/Ionisation of Fluorinated Fullerenes, Eur. J. Mass Spectrom., 1997, 3, 407–414 CrossRef.
- H. Steger, U. Mische, W. Kamke, A. Ding, M. Fieber-Erdmann and T. Drewello, Ionisation and Fragmentation Dynamics of Highly Fluorinated Fullerenes C60F46,48 and C70F54,56 after Excitation with Synchrotron Radiation, Chem. Phys. Lett., 1997, 276, 39–46 CrossRef CAS.
- M. P. Barrow, X. Feng, J. I. Wallace, O. V. Boltalina, R. Taylor, P. J. Derrick and T. Drewello, Characterization of Fullerenes and Fullerene Derivatives by Nanospray, Chem. Phys. Lett., 2000, 330, 267–274 CrossRef CAS.
- R. M. Danell, A. S. Danell, G. L. Glish and R. W. Vachet, The Use of Static Pressures of Heavy Gases Within a Quadrupole Ion Trap, J. Am. Soc. Mass Spectrom., 2003, 14, 1099–1109 CrossRef CAS PubMed.
- Á. Kuki, L. Nagy, A. Memboeuf, L. Drahos, K. Vékey, M. Zsuga and S. Kéki, Energy-Dependent Collision-Induced Dissociation of Lithiated Polytetrahydrofuran: Effect of the Size on the Fragmentation Properties, J. Am. Soc. Mass Spectrom., 2010, 21, 1753–1761 CrossRef PubMed.
- T. M. Kertesz, L. H. Hall, D. W. Hill and D. F. Grant, CE50: Quantifying Collision Induced Dissociation Energy for Small Molecule Characterization and Identification, J. Am. Soc. Mass Spectrom., 2009, 20, 1759–1767 CrossRef CAS PubMed.
- A. Memboeuf, A. Nasioudis, S. Indelicato, F. Pollreisz, Á. Kuki, S. Kéki, O. F. van den Brink, K. Vékey and L. Drahos, Size Effect on Fragmentation in Tandem Mass Spectrometry, Anal. Chem., 2010, 82, 2294–2302 CrossRef CAS PubMed.
- X. Guo, M. C. Duursma, P. G. Kistemaker, N. M. M. Nibbering, K. Vékey, L. Drahos and R. M. A. Heeren, Manipulating Internal Energy of Protonated Biomolecules in Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry, J. Mass Spectrom., 2003, 38, 597–606 CrossRef CAS PubMed.
- K. Vékey, Internal Energy Effects in Mass Spectrometry, J. Mass Spectrom., 1996, 31, 445–463 CrossRef.
- N. Vinokur and V. Ryzhov, Using Collision-Induced Dissociation with Corrections for the Ion Number of Degrees of Freedom for Quick Comparisons of Relative Bonding Strength, J. Mass Spectrom., 2004, 39, 1268–1274 CrossRef CAS PubMed.
- C. Lifshitz, Kinetic Shifts, Eur. J. Mass Spectrom., 2002, 8, 85–98 CrossRef CAS.
- A. K. Shukla and J. H. Futrell, Tandem Mass Spectrometry: Dissociation of Ions by Collisional Activation, J. Mass Spectrom., 2000, 35, 1069–1090 CrossRef CAS.
- J. M. Daniel, S. D. Friess, S. Rajagopalan, S. Wendt and R. Zenobi, Quantitative Determination of Noncovalent Binding Interactions Using Soft Ionization Mass Spectrometry, Int. J. Mass Spectrom., 2002, 216, 1–27 CrossRef CAS.
- Y.-L. Chen, J. M. Campbell, B. A. Collings, L. Konermann and D. J. Douglas, Stability of a Highly Charged Noncovalent Complex in the Gas Phase: Holomyoglobin, Rapid Commun. Mass Spectrom., 1998, 12, 1003–1010 CrossRef CAS PubMed.
- S. Jung, J. D. van Paauwe, P. D. W. Boyd and S. K. Shin, Noncovalent Endo-Binding of Fullerenes to Diprotonated Bisporphyrins, Phys. Chem. Chem. Phys., 2011, 13, 20248–20254 RSC.
- G. J. Van Berkel and F. Zhou, Observation of Gas-Phase Molecular Dications Formed from Neutral Organics in Solution via The Controlled-Current Electrolytic Process Inherent to Electrospray, J. Am. Soc. Mass Spectrom., 1996, 7, 157–162 CrossRef CAS PubMed.
- G. J. Van Berkel and F. Zhou, Electrospray as a Controlled-Current Electrolytic Cell: Electrochemical Ionization of Neutral Analytes for Detection by Electrospray Mass Spectrometry, Anal. Chem., 1995, 67, 3958–3964 CrossRef CAS.
- T. Drewello, C. B. Lebrilla, H. Schwarz and T. Ast, Charge Separation (Coulomb Explosion) Processes of Doubly Charged Cations of Sandwich Compounds in the Gas Phase: Evidence for the Junction of two C5H5 Units, J. Organomet. Chem., 1988, 339, 333–338 CrossRef CAS.
- R. Herzschuh and T. Drewello, The Fragmentation Dynamics of Small Cs(CsI)n+ Cluster Ions Under Low-Energy Multiple Collision Conditions, Int. J. Mass Spectrom., 2004, 233, 355–359 CrossRef CAS.
- Á. Révész, T. A. Rokob, G. Maász, L. Márk, H. Hevér, L. Drahos and K. Vékey, Fragmentation, Structure, and Energetics of Small Sodium Formate Clusters: Evidence for Strong Influence of Entropic Effects, Int. J. Mass Spectrom., 2013, 354–355, 292–302 CrossRef.
| This journal is © the Owner Societies 2015 |