Probing structural patterns of ion association and solvation in mixtures of imidazolium ionic liquids with acetonitrile by means of relative 1H and 13C NMR chemical shifts
Abstract
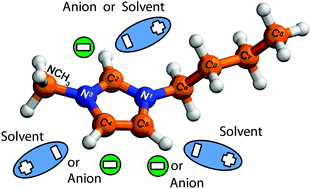
Mixtures of ionic liquids (ILs) with polar aprotic solvents in different combinations and under different conditions (concentration, temperature etc.) are used widely in electrochemistry. However, little is known about the key intermolecular interactions in such mixtures depending on the nature of the constituents and mixture composition. In order to systematically address the intermolecular interactions, the chemical shift variation of 1H and 13C nuclei has been followed in mixtures of imidazolium ILs 1-n-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4), 1-n-butyl-3-methylimidazolium hexafluorophosphate (BmimPF6), 1-n-butyl-3-methylimidazolium trifluoromethanesulfonate (BmimTfO) and 1-n-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (BmimTFSI) with molecular solvent acetonitrile (AN) over the entire composition range at 300 K. The concept of relative chemical shift variation is proposed to assess the observed effects on a unified and unbiased scale. We have found that hydrogen bonds between the imidazolium ring hydrogen atoms and electronegative atoms of anions are stronger in BmimBF4 and BmimTfO ILs than those in BmimTFSI and BmimPF6. Hydrogen atom at position 2 of the imidazolium ring is substantially more sensitive to interionic hydrogen bonding than those at positions 4–5 in the case of BmimTfO and BmimTFSI ILs. These hydrogen bonds are disrupted upon dilution in AN due to ion dissociation which is more pronounced at high dilutions. Specific solvation interactions between AN molecules and IL cations are poorly manifested.

 Please wait while we load your content...
Please wait while we load your content...