Investigating the molecular and aggregated states of a drug molecule rutaecarpine using spectroscopy, microscopy, crystallography and computational studies†
Abstract
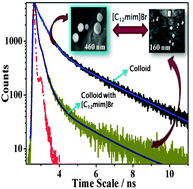
The photophysical properties of a potential drug molecule rutaecarpine have been investigated in molecular as well as aggregated states. All systems have been characterized by various spectroscopic, microscopic and dynamic light scattering (DLS) techniques. The investigation has been carried out by keeping the fact in mind that hydrophobic organic molecules have a strong tendency to form aggregates in aqueous solution. A blue shift in the absorption spectrum of rutaecarpine has been observed for aggregates (compared to molecular solution) indicating the formation of H-type aggregates. The intermolecular interactions responsible for such aggregation have been further investigated through crystallographic and computational studies. It has been observed that π–π stacking interactions among the monomer units play an important role in the formation of H-type aggregates. Quantum mechanical calculations also substantiate the blue shift in the absorption that has been observed for aggregates. In the present case, enhanced emission for aggregates as compared to the molecular solution of rutaecarpine has also been observed. The observed enhanced emission upon aggregation is attributed to the decrease of the non-radiative rate constant (knr) upon aggregation. The effect of a surface active ionic liquid (SAIL), 1-dodecyl-3-methylimidazolium bromide ([C12mim]Br), on the aggregation of rutaecarpine has been investigated. Interestingly, in addition to the decrease in the particle size, a change in the morphology of the aggregates has also been observed with gradual addition of [C12mim]Br to the colloidal solution of rutaecarpine. The present study demonstrates that a SAIL can effectively be used as a medium for dissociation of colloidal aggregates and encapsulation of molecular species, which in turn would be helpful in influencing the drug activity.

 Please wait while we load your content...
Please wait while we load your content...