Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultrafast coherent oscillations reveal a reactive mode in the ring-opening reaction of fulgides†
C.
Slavov
a,
N.
Bellakbil
a,
J.
Wahl
a,
K.
Mayer
b,
K.
Rück-Braun
b,
I.
Burghardt
a,
J.
Wachtveitl
a and
M.
Braun
*a
aInstitute of Physical and Theoretical Chemistry, Goethe University, Max-von-Laue-Str. 7, 60438 Frankfurt/Main, Germany. E-mail: braun@theochem.uni-frankfurt.de; Fax: +49 69 798 29709
bDepartment of Chemistry, Technical University Berlin, Strasse des 17. Juni 135, 10623 Berlin, Germany
First published on 29th April 2015
Abstract
The ultrafast ring-opening reaction of photochromic fulgides proceeds via conical intersections to the ground state isomers involving activation barriers in the excited state. The coherent oscillations observed in the femtosecond transient absorption signal of a methyl-substituted indolylfulgide were analysed in the framework of vibrational wavepackets to expose a dominant low-frequency mode at ∼80 cm−1. The quantum chemical calculations in the relaxed excited state geometry of this fulgide revealed that the experimentally observed vibrational normal mode has a dominant contribution to the relevant ring-opening reactive coordinate.
Introduction
The mounting interest from both industry and science for highly selective and efficient control of different (bio)physical processes and (bio)chemical reactions has placed an enormous demand on the research and development of molecular-based switches.1,2 The basic principle of operation of molecular switches is the reversible transition of the switching molecule between forms with different physicochemical properties.3,4 Photochromic switches, in which this transition is triggered by electromagnetic radiation,2 represent one of the widely employed groups of molecular switches. The utilization of light as a trigger for the switching process allows easy manipulation with a high degree of spatiotemporal control. This, combined with the nearly instantaneous property change of the molecule, gives photoswitches perhaps the greatest application potential.The fulgide family is a major group of photochromic compounds, which has attracted considerable attention since their discovery.5,6 Fulgides are derivatives of dimethylene-succinic anhydrides, substituted with an aromatic ring (Fig. 1) and depending on the aryl substitution they are termed phenyl-, furyl-, thienyl-, pyrryl- or indolylfulgides. The wide range of photochromic reactions observed in fulgides (fulgimides, fulgenates, or fulgenolides) is well described in a comprehensive review by Yokoyama.7 Substitution of an aromatic ring at the fulgenic acid leads to the formation of the well-known 1,3,5-hexatriene motif, which allows the 6π-electrocyclization reaction to cyclohexadiene (Fig. 1C). The photochromism of these switches is therefore mainly based on interconversion of the open (colourless hexatriene motif) and the closed (coloured cyclohexadiene motif) forms via ring-closing and ring-opening reactions. The open (cyclisable) isomer of the fulgide investigated here is termed E-form and the closed isomer as C-form. There exist two non-desired pathways, reducing the yield of the closing reaction: (i) the photochemical E/Z isomerization between the cyclisable open E-form and a noncyclisable open Z-form and (ii) the thermal enantiotopomerization along the single bond of the open hexatriene motif, leading to a fast racemization of the chiral molecules in the electronic ground state (GS). Both disturbing processes, E/Z isomerization and enantiotopomerization,8,9 require an extensive rotational movement and thus can be strongly reduced by bulky substituents.10,11 In contrast, the ring-opening/closing reaction is space-saving with a limited movement of side groups. In this respect, indolyl-substituted fulgides show a very low contribution (typically <10%) of noncyclisable open form molecules in the photostationary state (PSS) and an increased barrier for GS enantiotopomerization.12 Therefore we have focused the current study on investigating the ring-opening reactions of an indolylfulgide derivative.
Indolylfulgides were investigated by several groups before.8,13–15 These molecules feature thermally stable GS isomers, thus structural changes can be initiated exclusively by optical excitation.7 The photochemical stability is very high and they can be switched between the open and closed forms several thousand times without significant degradation.16 Moreover, the extension of the closed and open form absorption spectra into the visible spectral range enables UV-free switching experiments, which is advantageous especially for biological applications.17 A major problem here is the relatively low stability of indolylfulgides in aqueous solution, which is due to the high reactivity of the succinic anhydride ring towards protic solvents. Nevertheless, there are first studies demonstrating that the stability of these compounds in protic solvents can be improved by replacing the succinic anhydride ring with a succinimide ring.18
The ring-opening,19–22 the ring-closing14,19 as well as the E/Z isomerization8,9 reactions of fulgides were studied in the last years with femtosecond time resolution. After photoexcitation of the C-form in the Franck–Condon (FC) region of the first singlet ES (S1) the molecule promptly relaxes to a local minimum on the S1 potential energy surface (PES). At least two conical intersections to the GS23,24 are accessible via barriers on the S1 PES, which leads to ring-opening reaction times of a few picoseconds. It was observed that this reaction depends strongly on the experimental conditions – solvent polarity, temperature (activated process), even excitation wavelength (spectral tuning in the S1 absorption band).22,25 Furthermore, a strong increase in the quantum efficiency (QE) and the reaction speed is observed after excitation in the higher electronic states, which clearly shows that different reaction pathways are accessible.26 In contrast, the ring-closing is totally robust against external factors (e.g. solvent (polarity), temperature, or excitation wavelength).27 The studies show that changes in these factors do not influence the observed dynamics and QE; no indication for an activated behaviour is detected. The ring-closing reaction is ultrafast and proceeds typically in the sub-ps time-range. In summary, the dominant photochromic reactions occurring in indolylfulgides (ring-opening and ring-closing) are ultrafast and occur without involvement of long-lived intermediates.
We focus here on the excited state (ES) properties of the C-form of a methyl-substituted indolylfulgide (MIF), monitored by femtosecond transient absorption (TA) spectroscopy as a function of solvent polarity and excitation wavelength. The vibrational wavepacket motion, observed in the TA data is discussed within the frame of quantum chemical calculations. The investigation is supported by a thorough steady-state characterization in dependence of solvent polarity, excitation wavelength and temperature.
Materials and methods
Sample preparation
The synthesis of the investigated methyl substituted indolylfulgide (MIF), has been previously described in detail.28 The so-synthesized MIF in pure crystalline E-isomer form was dissolved for the experiments either in benzene (Sigma-Aldrich) or in acetonitrile (VWR International). The extinction coefficients of interest are given in the caption of Fig. 1. The ratio of the different forms, Z/E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, in the PSSUV (405 nm) in benzene was determined using HPLC to be 19
C, in the PSSUV (405 nm) in benzene was determined using HPLC to be 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81.
81.
Steady-state spectroscopy and QE determination
Absorption spectra were recorded on a Specord S600 (Analytik Jena) absorption spectrometer using a fused silica cuvette with 1 cm optical path length. The temperature dependence (10–50 °C) of the QE was determined using a home-built thermostated holder, which can be installed directly in the absorption spectrometer. The holder is equipped with connectors for attaching optical fibers guiding to the sample illumination light from mounted high-power LEDs (ThorLabs, M365L2, M385L2, M530L2, M565L2, driver DC4100). The light from the optical fibers emerges directly at the surface of the cuvette with the sample, which ensures that the sample is illuminated by the complete output of the fiber. The intensity of the light coming out of the optical fiber was determined using a calibrated light detector (P-9710, Gigahertz-Optik). The PSSVIS (530 nm or 565 nm illumination) and the PSSUV (365 nm or 385 nm illumination) were always prepared at 20 °C and the sample was continuously stirred using a magnetic stirrer (Thermo Scientific). The induced absorption changes at 530 nm for both the ring-opening and the ring-closing reaction were monitored for 15–60 min, depending on the conversion rate of the given sample. For the calculation of the QE only the initial change of the absorbance (first few min) was used (cf. ESI,† Fig. S1). During this period, the photons are absorbed only by the reactant as there is negligible amount of product formed and thus the absorbance changes are in linear dependence with the amount of absorbed light. To minimize the effect of the spectrometer measuring light on the QE determination an optical filter was used to cut active spectral contributions.Quantum efficiency calculation
The photochemical QE is defined as the ratio of the reacted molecules (Nreact) and the number of absorbed photons (Nabs). Nreact for a photochromic compound with thermally stable isomers can be determined by the associated absorbance changes (ΔOD) in a range specific for the product or the reactant: | (1) |
The Nabs can be calculated via:
 | (2) |
Thus the QE can be calculated by the following equation:
 | (3) |
VIS–pump–probe spectroscopy
The time-resolved TA measurements were performed using a home-built pump–probe setup. Ultrashort laser pulses (150 fs) were provided by an oscillator–amplifier system (Clark, MXR-CPA-iSeries) operating at a repetition rate of 1 kHz (775 nm). The pump pulses between 500 and 570 nm (see text and figures for specific excitation wavelength) were generated in a home-built two stage NOPA (non-collinear optical parametric amplifier)29,30 and focused to a diameter of ∼200 μm at the sample position. A prism compressor placed between the two NOPA stages was used to compress the pump pulses to about 60–80 fs (determined with a PulseCheck USB 15 ShortPulse Autocorrelator, APE). The pulse energy was adjusted to ensure that <10% of the molecules are excited per pulse. Single filament white light (WL) pulses covering a spectral range between 300 nm and 700 nm were generated by focusing the laser fundamental in a 5 mm thick CaF2 crystal. The WL pulses were split into two parts – probe and reference. The reference beam was guided directly to a spectrograph, while the probe beam was focused to a diameter of ∼100 μm at the sample position and then collected and guided to a second spectrograph. Both spectrographs (AMKO Multimode) were equipped with 1200 grooves per mm gratings blazed at 500 nm and a photodiode array (PDA) combined with a signal processing chip (Hamamatsu Photonics, S8865-64) and a driver circuit (Hamamatsu Photonics, C9118), which allows single shot detection with kHz rate. The analog PDA signals were digitized at 16 bits by a data acquisition card (National Instruments, NI-PCI-6110). The detection range was set to 420–650 nm with a spectral resolution of 3.6 nm. The experiments were performed under magic angle conditions (54.7° pump–probe polarization angle difference) to eliminate anisotropic contributions. The instrument response function (IRF) was typically in the range of 80 fs. The sample was contained in a fused silica cuvette with 1 mm optical path length. The cuvette was continuously moved in the plane perpendicular to the direction of probe pulse propagation. The cuvette was continuously illuminated with a high-power 385 nm LED (ThorLabs, M385L2) to keep the sample in the PSSUV.Data analysis
The time-resolved data were analysed by means of Global Lifetime Analysis (GLA),31,32 which represents simultaneous analysis of all transients at different detection wavelengths with a single set of exponential functions. Furthermore, the data were subjected to Lifetime Density Analysis (LDA),32 where the pre-exponential amplitudes in a sum of a large number (∼100) of exponential functions with fixed, equally spaced (on a decimal logarithm scale) lifetimes are determined. In contrast to GLA, LDA is a model independent type of data analysis that naturally deals with non-exponential or stretched exponential kinetics.32,33 The LDA recovers the lifetime distribution at each detection wavelength and thus the results can be presented in the form of a contour map called Lifetime Density Map (LDM). The coherent artifact contribution in the transient signals at time zero position was approximated with a function composed of a Gaussian and/or its first and second derivative32,34 and fitted within the same routine as the GLA and LDA. The data analysis was performed using OPTIMUS a program recently developed in our group.32Quantum chemical calculations
The quantum chemical calculations were performed with the help of TURBOMOLE v6.435 using the TDDFT BHLYP functional36–40 and the 6-31G* basis set.41,42Results and discussion
Stationary spectroscopy and QE determination
The structures of the different forms of the MIF studied here are illustrated in Fig. 1C. The stationary absorption spectra of the pure open E-form dissolved in benzene and acetonitrile as well as the corresponding PSSs generated after 365 nm illumination are shown in Fig. 1A and B. The PSSs show absorption bands in the visible spectral range characteristic for the closed C-form. The change in solvent polarity from the non-polar benzene to the polar acetonitrile leads to a bathochromic shift of the C-form band from 535 nm to 547 nm. Illumination of the C-form with visible light (530 nm LED) induces the reverse reaction. The stability of the compounds in the two solvents was evaluated by a series of UV-VIS illumination cycles. The illumination was performed until a PSS is achieved and absorbance changes were measured at 530 nm (ESI,† Fig. S2). After 18 cycles the absorbance of the MIF in benzene is changed only slightly (2.5%), while in acetonitrile the absorbance is reduced by 15% in only 9 cycles. The decrease of the photostability of fulgides in aprotic polar solvents was reported previously and attributed to degradation due to hydrogen migration followed by tautomerization.17The temperature dependence of the QE of the ring-opening and the ring-closing reactions was evaluated in the range of 10–50 °C. The ring-closing reaction was initiated by illumination at 365 and 385 nm, while the ring-opening reaction was initiated with 530 and 565 nm. The results are summarized in Table 1. The QE of the formation of the closed form in benzene is ∼14–15%, while in acetonitrile it is reduced to ∼5%. Within the error limits of the experiments, the QE of the ring-closing reaction does not show a significant temperature or excitation wavelength dependence (Table 1). However, the results show a clear solvent dependence with a 3-fold decrease of the QE from non-polar (benzene) to polar (acetonitrile) solvents. The QEs of the ring-opening reaction show an even stronger decrease (∼5-fold) with the increase of solvent polarity (Table 1). Furthermore, in contrast to the ring-closing, the ring-opening is characterized by pronounced temperature and excitation wavelength dependence (Table 1). The temperature dependent increase of the QE of the ring-opening reaction in benzene is ∼28% for 530 nm illumination and ∼34% for 565 nm illumination. In acetonitrile the temperature dependence of the QE is even stronger (Table 1) showing a ∼3-fold increase. In benzene the excitation wavelength dependent (565 nm → 530 nm) increase of the QE of the ring-opening reaction is in the range of ∼30%, while in acetonitrile it is less distinct. Similar dependence of the QE of the ring-opening reaction has been observed previously for both indolylfulgides and indolylfulgimides.25 The excitation wavelength dependence of the QE for the ring-opening reaction is due to the presence of an energetic barrier in the S1 ES. Such a barrier is easier to overcome when more optical excess energy is available after excitation with shorter wavelengths and for ultrafast reactions occurring before the dissipation of this excess energy to the environment. Assuming knon-reactive ≫ kreactive, the temperature dependence of the QE should be governed by the reactive rate and show an Arrhenius dependence.43 Hence, we were able to calculate the activation energy, Ea, for the ring-opening photoreaction, which in the case of MIF in benzene is ∼400 cm−1 (∼4.8 kJ mol−1), while in acetonitrile is about four times higher, ∼1700 cm−1 (∼20 kJ mol−1). See Fig. S3 (ESI†) for the Arrhenius plots.
| [°C] | QE of ring-closing, [%] ΦUVE→C | QE of ring-opening, [%] ΦVISC→E | ||||||
|---|---|---|---|---|---|---|---|---|
| Benzene [nm] | Acetonitrile [nm] | Benzene [nm] | Acetonitrile [nm] | |||||
| 365 | 385 | 365 | 385 | 530 | 565 | 530 | 565 | |
| 10 | 15.2 | 14.7 | 5.1 | 5.3 | 6.3 | 4.9 | 0.57 | 0.54 |
| 20 | 14.7 | 14.1 | 4.8 | 5.2 | 6.8 | 5.1 | 1.0 | 0.83 |
| 30 | 14.5 | 14.8 | 5.5 | 5.1 | 7.1 | 5.6 | 1.2 | 1.0 |
| 40 | 14.3 | 13.5 | 5.0 | 5.1 | 7.7 | 6.1 | 1.6 | 1.3 |
| 50 | 13.9 | 14.7 | 5.1 | 5.0 | 8.1 | 6.6 | 1.9 | 1.5 |
Ultrafast transient absorption
A series of TA experiments with <100 fs resolution was performed to determine the solvent and excitation wavelength dependence of the ultrafast ring-opening C → E reaction of the MIF. In Fig. 2A and C the TA data of the sample in benzene and acetonitrile after excitation at the corresponding absorption maxima (538 and 555 nm) are shown (cf. ESI,† Fig. S4 for a complete overview). The coherent artifact contributions in the transient data at delay times ∼0 ps were fitted and subtracted from the experimental data for clearer representation.32 In addition, the delay time zero dispersion was also corrected.31,32 The TA data of MIF in benzene and in acetonitrile are characterized by the presence of pronounced excited state absorption (ESA) over the whole detection wavelength range. In acetonitrile, three distinct ESA bands (<440 nm, ∼500 nm and ∼600 nm) can be observed, while in benzene only two (∼460 nm and ∼620 nm) are visible. The lack of a third ESA band in benzene is due to the strong overlap between the ESA and the GS bleach (GSB) signals in the 450–650 nm range, which diminishes the transient signal in this region. The ESA bands in acetonitrile appear to be blue-shifted by about 10–20 nm as compared to benzene. The red-most, strongest band in both solvents (600 nm in acetonitrile and 620 nm in benzene) shows a clear time-dependent blue shift on the time scale of a few ps. The open-form product formation is discernible only in benzene at delay times longer than 10 ps by the remaining GSB signal of the C-form (500–600 nm). In acetonitrile, this GSB contribution is negligible due to the low conversion QE (Table 1). Qualitatively, the results for different excitation wavelengths in the S0 → S1 absorption band appear to be very similar (Fig. S4, ESI†). | ||
| Fig. 2 (left side) TA data of MIF C-form in (A) benzene and (C) acetonitrile; (right side) DAS obtained from the GLA of the TA data of MIF C-form in (B) benzene and (D) acetonitrile. For a complete overview of the excitation wavelength dependence please see ESI,† Fig. S4 and S5. | ||
Global lifetime analysis (GLA) of the time-resolved data
The TA data were analysed by means of GLA31,32 to determine the main lifetime components contributing to the observed kinetics. The decay-associated spectra (DAS) obtained from the GLA of the transient data from MIF in benzene and acetonitrile after excitation in their corresponding S0 → S1 absorption maximum are shown in Fig. 2B and D and a summary for all excitation wavelengths is shown in Fig. S5 (ESI†) and in Table 2. The fastest lifetime component, τ1, (Fig. 2B and D and Fig. S5, ESI†) shows a typical positive-negative amplitude feature in the 550–650 nm range where the main ESA band is located. This effect describes the time-dependent blue shift of the ESA band (positive amplitude in the DAS – decay of ESA, negative amplitude – rise of ESA). The DAS of the second lifetime component is positive over the whole detection wavelength range and accounts for the overall decay of the ESA. The infinite spectrum shows the remaining bleach of the initial absorption of the C-form and thus reports on the amount of product (open-form) formation. A clear difference in the infinite spectrum of the MIF in benzene and in acetonitrile is the lack of GSB feature in the acetonitrile spectra reflecting the very low conversion QE.| MIF in benzene | MIF in acetonitrile | ||||||
|---|---|---|---|---|---|---|---|
| λ exc [nm] | τ 1 [ps] | τ 2 [ps] | τ 3 [ps] | λ exc [nm] | τ 1 [ps] | τ 2 [ps] | τ 3 [ps] |
| 505 | 1.74 | 6.70 | Inf | 520 | 0.73 | 14.32 | Inf |
| 538 | 1.90 | 7.31 | Inf | 555 | 0.67 | 14.24 | Inf |
| 555 | 2.01 | 7.58 | Inf | 570 | 0.85 | 14.61 | Inf |
The excitation wavelength dependence of the shape and the spectral position of the DAS is very weak for MIF in both solvents. However, the amplitudes do show a trend of decrease with the shift of the excitation wavelength to the red side of the corresponding absorption spectra. This decrease cannot be assigned solely to a change in the excitation energy, as this energy was roughly the same for all experiments. Furthermore, a subtle excitation wavelength dependence trend, which results in shorter lifetimes for higher excitation photon energies, can also be observed for the lifetimes (Table 2).
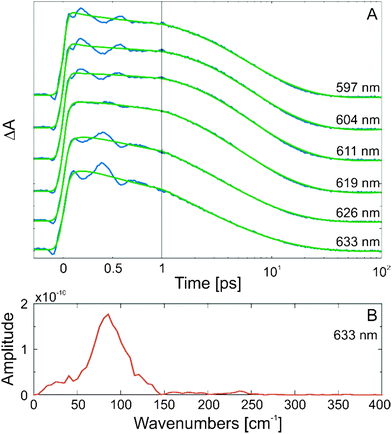
Coherent oscillations
On the sub-ps timescale, the TA data of MIF are characterized by the presence of strong signal modulation in the ESA bands in both solvents (benzene and acetonitrile). These signal oscillations are particularly distinct above 560 nm (Fig. 2 and Fig. S4, ESI†) and persevere until ∼0.8–1 ps. A comparison of the TA data at a wavelength within the ESA (580–640 nm) band containing the time-dependent signal modulation pattern with the fit yielded from the GLA is shown in Fig. 3A. The Fourier transform of the residuals resulting from the subtraction of the fit from the experimental data allowed extraction of the frequency of the signal modulation. An example of the frequency spectra is shown in Fig. 3B. The main oscillation frequency appears to be ∼80 cm−1, although contribution from other frequencies in this range cannot be excluded.Excited state relaxation dynamics
The photochemical ring-opening reaction of MIF occurs with the transition from the electronic ES to the GS (S1 → S0) and is reflected by the decay of the ESA signal in the TA data. The GLA of the ultrafast time-resolved data shows that the dominant ESA decay (all-positive DAS, Fig. 2) is characterized by ∼7 ps lifetime in benzene and by ∼14 ps lifetime in acetonitrile. Our theoretical calculations (see Experimental section) yielded a dipole moment of MIF in the GS state of ∼6.7 D, which grows to ∼12.1 D in the S1 FC point and decreases to ∼11.3 D in the relaxed S1 state. This change in dipole moment leads to an energetic stabilization of the S1 state in polar surrounding, which is in agreement with the red shift of the steady-state absorption maximum (Fig. 1) and the corresponding blue shift of the ESA maximum (Fig. 2) in acetonitrile. The S1 state stabilization has two consequences. On the one hand, it leads to an increase of the ES reaction barrier (400 cm−1 in benzene, and ∼1700 cm−1 in acetonitrile) as confirmed by the quantum efficiency experiments (Table 1). On the other hand, we have shown for a similar compound that the internal conversion pathway is thermally activated22, thus a stabilization of the S1 state in polar solvents will also increase this energetic barrier. In effect, the polar solvent induced stabilization of the S1 state leads to an increase of the ES lifetime of the MIF C-form as observed in our ultrafast experiments (∼7 ps in benzene, ∼14 ps in acetonitrile). No picosecond GS cooling dynamics, as the one observed in other fulgide compounds,21 has been resolved by the GLA of the MIF TA data. The lack of an additional GS cooling lifetime component is due to the temporal overlap of this process with the dominant ES relaxation signal.On the earlier timescale, the TA changes are associated with dynamics on the ES PES. The DAS of the shorter lifetime component (∼1.9 ps in benzene and ∼0.7 ps in acetonitrile) shows a positive–negative amplitude feature (Fig. 2, >560 nm), characteristic for relaxation from the FC region. The strong solvent dependence of this FC relaxation lifetime (nearly 3 times shorter in acetonitrile as compared to benzene) hints at significant involvement of ES-induced solvent reorganization dynamics.
The GLA of the TA data of the MIF C-form indicated a potential contribution in the ultrafast dynamics by a <0.5 ps lifetime component. However, since the processes (wavepacket motion, vibrational energy redistribution, and solvation dynamics) occurring at this ultrafast timescale have non-exponential nature, it is hard to unambiguously assign the lifetime components obtained from GLA. Henceforth, to confirm the presence of even faster dynamics in the TA data of the ring-opening reaction of MIF, we have performed a model-independent lifetime density analysis (LDA).32 The results from the LDA are shown in Fig. 4 (cf. Fig. S6 and S7, ESI† for details) in the form of lifetime density maps (LDMs). Indeed, the LDMs show ∼100–200 fs lifetime distribution features, particularly pronounced for the ring-opening reaction of MIF in benzene (Fig. 4A, >570 nm). The same lifetime distributions appear to be shifted to even shorter lifetimes in acetonitrile (Fig. 4B and Fig. S6, ESI†) in line with the previously discussed FC relaxation solvent dependence. The shortest lifetime component, τ1, in the GLA (Fig. 2B and D) is associated with the lifetime distributions located at ∼1 ps in benzene (Fig. 4A and Fig. S5, ESI†) and at ∼500–600 fs in acetonitrile (Fig. 4B and Fig. S6, ESI†). Interestingly, the wavelength shift between the ∼100 fs lifetime distributions and the ∼0.5–1.5 ps lifetime distributions (Fig. 4, >570 nm) is significant in benzene but not so pronounced in acetonitrile. The difference in the energetics between those two lifetime distributions is indicative of the presence of a quasi-two step relaxation reaction in the S1 ES. The first step could tentatively be assigned to the relaxation of the MIF from the FC region, while the second step should be linked to changes of the S1 PES due to solvent reorganization.
The LDMs are dominated by the broad positive lifetime distributions on the picosecond lifetime scale, which account for the decay of the ESA and thus for the photochemical reaction of ring-opening. The positive picosecond lifetime distributions (>2 ps lifetime in Fig. 4 and Fig. S5 and S6, ESI†) show a strongly elongated shape, which could be associated with the time overlap of the ES relaxation and the GS cooling processes.
Excited state coherent dynamics
The strong coherent oscillations of the TA signal from the ring-opening reaction of MIF appear at the position of the strongest ESA band (in the 560–640 nm range, Fig. 2 and Fig. S3, ESI†). The oscillatory pattern shows a phase shift with a zero position at the maximum of the ESA band (Fig. 3A) and thus the coherent oscillations can be assigned straightforwardly to wavepacket motion on the ES PES of the MIF C-form. Such a wavepacket dynamics has been previously observed for similar compounds9,25,44 but has not been studied in detail. Here, we have analysed the coherent oscillations of the TA signal and obtained the frequency spectrum (Fig. 3B). The spectrum is centred at about ∼80 cm−1 in benzene and is slightly blue shifted in acetonitrile. Nevertheless, the band covers a relatively broad frequency range from ∼40 to ∼150 cm−1. To determine whether higher frequency coherent oscillations are potentially detectable at the given experimental limits, a damping curve (Fig. 5A) for TA signal oscillations was simulated by convoluting a 80 fs FWHM IRF with cosine functions of different frequencies.45 The result of this simulation indicates that in our TA experiment only coherent oscillations with frequencies below ∼200 cm−1 are detectable.In an attempt to assign the experimentally observed coherent oscillation to a specific molecular vibration, quantum chemical calculations using TURBOMOLE35 were performed for the S0 minimum  and the S1 minimum
and the S1 minimum  structures. In addition, the energy of the FC point and the vibrational normal modes for the
structures. In addition, the energy of the FC point and the vibrational normal modes for the  structure were also calculated. MIF comprises 39 atoms and thus 111 vibrational modes (νi) and their respective displacement vectors (xi). Since the oscillatory wavepacket motion is observed in the ESA TA signal, we calculated which normal modes induce the largest shift,
structure were also calculated. MIF comprises 39 atoms and thus 111 vibrational modes (νi) and their respective displacement vectors (xi). Since the oscillatory wavepacket motion is observed in the ESA TA signal, we calculated which normal modes induce the largest shift,  , in the respective transition energies (cf.Fig. 5A and Fig. S8, ESI†).46 In the low frequency range, accessible within the time resolution of our experiments, the ∼80 cm−1 mode causes the largest shift in accord with the experimentally observed coherent oscillation (Fig. 3B).
, in the respective transition energies (cf.Fig. 5A and Fig. S8, ESI†).46 In the low frequency range, accessible within the time resolution of our experiments, the ∼80 cm−1 mode causes the largest shift in accord with the experimentally observed coherent oscillation (Fig. 3B).
Evidently, significant amount of excitation energy is deposited in the ∼80 cm−1 mode of the molecule, however, some of this energy could also be redistributed into other normal modes during the relaxation from the  to the
to the  geometry. A measure of the excitation energy distribution over the different normal modes can be obtained by projecting the difference vector of these two geometries
geometry. A measure of the excitation energy distribution over the different normal modes can be obtained by projecting the difference vector of these two geometries  onto the basis set of the displacement vectors of the relaxed S1 vibrational normal modes (xi) (see Fig. 5B). From this analysis, we find that there are a number of modes that are being populated during the
onto the basis set of the displacement vectors of the relaxed S1 vibrational normal modes (xi) (see Fig. 5B). From this analysis, we find that there are a number of modes that are being populated during the  relaxation. However, the highest amount of energy is distributed again into the ∼80 cm−1 mode.
relaxation. However, the highest amount of energy is distributed again into the ∼80 cm−1 mode.
Our results clearly indicate that the experimentally observed ∼80 cm−1 mode plays a dominant role in the ES dynamics of MIF. Thus, the question arises whether this mode also participates in the photochemical ring-opening reaction. Previous theoretical studies23,47 on the reactive coordinates involved in a ring-opening reaction have demonstrated that the critical coordinates defining the reaction are the dihedral angle, φ, accounting for distortion of the molecule from planarity, and the length of the diagonal (r) as defined in the inset of Fig. 5C for MIF. To verify whether the ∼80 cm−1 mode plays also a role in the ring-opening reaction we calculated the abovementioned dihedral angles and distances for each normal mode of the molecule. Since the coordinates of those modes are given by TURBOMOLE in Cartesian normalized units appropriate normalization is necessary to correctly evaluate the effect of a given mode on the structure of the molecule. Thus we calculated a reduced mass (μ) and frequency (ω) weighted displacement scaling (DS) factor (eqn (4), Fig. 5A).35 We used this factor to scale the relative dihedral angle change (φ) and the distance changes (r1 and r2) in Fig. 5C.
 | (4) |
In our theoretical analysis, we have examined three different factors that contribute to the amplitude of the vibrational wavepacket observed in the TA data: (i) temporal resolution (Fig. 5A) (ii) influence of different normal modes on the ESA transition wavelength (Fig. 5A) (iii) energy distribution into the different vibrational normal modes during the relaxation from the FC region (Fig. 5B). In all three cases the ∼80 cm−1 vibrational mode has a dominant impact on the observed TA signal. Independently, our calculations (Fig. 5C) showed that this mode also possesses the highest contribution to structural changes that relate to the reactive coordinates.
Conclusions
Based on our results we propose the following molecular mechanism for the ring-opening reaction (Fig. 6). After excitation of the C-form of the MIF compound in the FC region the molecule undergoes an ultrafast intramolecular relaxation (∼100 fs, Fig. 4), which is followed by a sub-ps solvent reorganization dynamics (Fig. 3 and 4). After this initial relaxation in the ES, the molecule is stabilized and needs to overcome an energetic barrier to reach the CI leading to the GS isomers. Furthermore, the observed coherent oscillation (wavepacket) of the TA signal, which according to our theoretical analysis can be assigned to an out-of-plane movement of the indolyl and the succinic acid rings, has a pronounced contribution to the reactive coordinates of the ring-opening reaction (Fig. 5C and ref. 23). This coherent motion can drive the molecule over the energetic barrier and to the CI. In this respect, we present an experimental evidence for a previously proposed theoretical reaction mechanism,23 which opens the road to targeting via chemical modification the active vibrational mode for achieving higher switching efficiency or manipulating the reaction rate as suitable for specific application.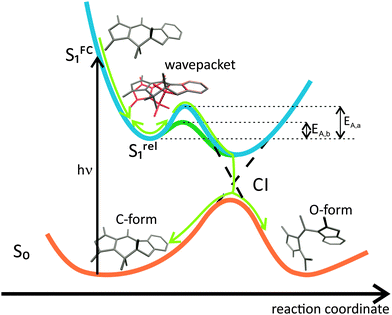 | ||
| Fig. 6 Reaction scheme for the ring-opening of the MIF C-form. See also Fig. S9 (ESI†). | ||
Acknowledgements
We thank Dr Miquel Huix-Rotllant for insightful discussions. Chavdar Slavov and Josef Wachtveitl acknowledge DFG (Grant WA 1850/4-1).References
- B. L. Feringa, W. F. Jager and B. Delange, Tetrahedron, 1993, 49, 8267–8310 CrossRef CAS.
- H. Bouas-Laurent and H. Dürr, Pure Appl. Chem., 2001, 73, 639–665 CrossRef CAS.
- G. H. Brown, Photochromism, Wiley, New Yourk, 1971 Search PubMed.
- Photochromism: Molecules and Systems, ed. H. Dürr and H. Bouas-Laurent, Elsevier, Amsterdam, 2003 Search PubMed.
- H. Stobbe, Ber. Dtsch. Chem. Ges., 1905, 38, 3673–3682 CrossRef CAS PubMed.
- H. Stobbe, Ber. Dtsch. Chem. Ges., 1907, 40, 3372–3382 CrossRef CAS PubMed.
- Y. Yokoyama, Chem. Rev., 2000, 100, 1717–1739 CrossRef CAS PubMed.
- F. Renth, M. Foca, A. Petter and F. Temps, Chem. Phys. Lett., 2006, 428, 62–67 CrossRef CAS PubMed.
- R. Siewertsen, F. Renth, F. Temps and F. Sönnichsen, Phys. Chem. Chem. Phys., 2009, 11, 5952–5961 RSC.
- S. Uchida, S. Yamada, Y. Yokoyama and Y. Kurita, Bull. Chem. Soc. Jpn., 1995, 68, 1677–1682 CrossRef CAS.
- M. A. Wolak, C. J. Thomas, N. B. Gillespie, R. R. Birge and W. J. Lees, J. Org. Chem., 2003, 68, 319–326 CrossRef CAS PubMed.
- Y. Yokoyama, Y. Shimizu and S. Uchida, J. Chem. Soc., Chem. Commun., 1995, 785–786 RSC.
- A. Santiago and R. S. Becker, J. Am. Chem. Soc., 1968, 90, 3654–3658 CrossRef CAS.
- M. Handschuh, M. Seibold, H. Port and H. C. Wolf, J. Phys. Chem. A, 1997, 101, 502–506 CrossRef CAS.
- B. Otto and K. Rück-Braun, Eur. J. Org. Chem., 2003, 2409–2417 CrossRef CAS PubMed.
- N. I. Islamova, X. Chen, S. P. Garcia, G. Guez, Y. Silva and W. J. Lees, J. Photochem. Photobiol., A, 2008, 195, 228–234 CrossRef CAS PubMed.
- Y. Yokoyama and K. Takahashi, Chem. Lett., 1996, 1037–1038 CrossRef CAS.
- N. I. Islamova, X. Chen, J. A. DiGirolamo, Y. Silva and W. J. Lees, J. Photochem. Photobiol., A, 2008, 199, 85–91 CrossRef CAS PubMed.
- H. Port, P. Gärtner, M. Hennrich, I. Ramsteiner and T. Schock, Mol. Cryst. Liq. Cryst., 2005, 430, 15–21 CrossRef CAS PubMed.
- F. O. Koller, W. J. Schreier, T. E. Schrader, A. Sieg, S. Malkmus, C. Schulz, S. Dietrich, K. Rück-Braun, W. Zinth and M. Braun, J. Phys. Chem. A, 2006, 110, 12769–12776 CrossRef CAS PubMed.
- S. Malkmus, F. O. Koller, B. Heinz, W. J. Schreier, T. E. Schrader, W. Zinth, C. Schulz, S. Dietrich, K. Rück-Braun and M. Braun, Chem. Phys. Lett., 2006, 417, 266–271 CrossRef CAS PubMed.
- S. Draxler, T. Brust, S. Malkmus, J. A. DiGirolamo, W. J. Lees, W. Zinth and M. Braun, Phys. Chem. Chem. Phys., 2009, 11, 5019–5027 RSC.
- D. Geppert, L. Seyfarth and R. de Vivie-Riedle, Appl. Phys. B: Lasers Opt., 2004, 79, 987–992 CrossRef CAS PubMed.
- A. Nenov, W. J. Schreier, F. O. Koller, M. Braun, R. de Vivie-Riedle, W. Zinth and I. Pugliesi, J. Phys. Chem. A, 2012, 116, 10518–10528 CrossRef CAS PubMed.
- T. Brust, S. Malkmus, S. Draxler, S. A. Ahmed, K. Rück-Braun, W. Zinth and M. Braun, J. Photochem. Photobiol., A, 2009, 207, 209–216 CrossRef CAS PubMed.
- T. Cordes, S. Malkmus, J. A. DiGirolamo, W. J. Lees, A. Nenov, R. de Vivie-Riedle, M. Braun and W. Zinth, J. Phys. Chem. A, 2008, 112, 13364–13371 CrossRef CAS PubMed.
- T. Cordes, T. T. Herzog, S. Malkmus, S. Draxler, T. Brust, J. A. DiGirolamo, W. J. Lees and M. Braun, Photochem. Photobiol. Sci., 2009, 8, 528–534 CAS.
- K. Rück-Braun, M. Å. Petersen, F. Michalik, A. Hebert, D. Przyrembel, C. Weber, S. A. Ahmed, S. Kowarik and M. Weinelt, Langmuir, 2013, 29, 11758–11769 CrossRef PubMed.
- T. Wilhelm, J. Piel and E. Riedle, Opt. Lett., 1997, 22, 1494–1496 CrossRef CAS.
- E. Riedle, M. Beutter, S. Lochbrunner, J. Piel, S. Schenkl, S. Spörlein and W. Zinth, Appl. Phys. B, 2000, 71, 457–465 CrossRef CAS.
- I. H. M. van Stokkum, D. S. Larsen and R. van Grondelle, Biochim. Biophys. Acta, Bioenerg., 2004, 1657, 82–104 CrossRef CAS PubMed.
- C. Slavov, H. Hartmann and J. Wachtveitl, Anal. Chem., 2015, 87, 2328–2336 CrossRef CAS PubMed.
- P. Trojanowski, J. Plötner, C. Grünewald, F. F. Graupner, C. Slavov, A. J. Reuss, M. Braun, J. W. Engels and J. Wachtveitl, Phys. Chem. Chem. Phys., 2014, 16, 13875–13888 RSC.
- S. A. Kovalenko, A. L. Dobryakov, J. Ruthmann and N. P. Ernsting, Phys. Rev. A: At., Mol., Opt. Phys., 1999, 59, 2369–2384 CrossRef CAS.
- TURBOMOLE V6.4 2012, A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, 2007 Search PubMed.
- P. A. M. Dirac, Proc. R. Soc. London, Ser. A, 1929, 123, 714–733 CrossRef CAS.
- J. Slater, Phys. Rev., 1951, 81, 385–390 CrossRef CAS.
- A. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS.
- C. Lee, W. Yang and R. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS PubMed.
- J. S. Binkley, J. A. Pople and W. J. Hehre, J. Am. Chem. Soc., 1980, 102, 939–947 CrossRef CAS.
- M. S. Gordon, J. S. Binkley, J. A. Pople, W. J. Pietro and W. J. Hehre, J. Am. Chem. Soc., 1982, 104, 2797–2803 CrossRef CAS.
- T. Brust, S. Draxler, S. Malkmus, C. Schulz, M. Zastrow, K. Rück-Braun, W. Zinth and M. Braun, J. Mol. Liq., 2008, 141, 137–139 CrossRef CAS PubMed.
- T. Brust, S. Draxler, A. Popp, X. Chen, W. J. Lees, W. Zinth and M. Braun, Chem. Phys. Lett., 2009, 477, 298–303 CrossRef CAS PubMed.
- S. Malkmus, R. Dürr, C. Sobotta, H. Pulvermacher, W. Zinth and M. Braun, J. Phys. Chem. A, 2005, 109, 10488–10492 CrossRef CAS PubMed.
- F. Schweighöfer, L. Dworak, M. Braun, M. Zastrow, J. Wahl, I. Burghardt, K. Rück-Braun and J. Wachtveitl, Sci. Rep., 2015, 5, 9368 CrossRef PubMed.
- A. Hofmann and R. de Vivie-Riedle, J. Chem. Phys., 2000, 112, 5054–5059 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/c5cp01878a |
| This journal is © the Owner Societies 2015 |