Open Access Article
Open Access ArticleStability and ionic mobility in argyrodite-related lithium-ion solid electrolytes†
Hao Min
Chen
,
Chen
Maohua
and
Stefan
Adams
*
Department of Materials Science and Engineering, National University of Singapore, Singapore 117575, Singapore. E-mail: mseasn@nus.edu.sg
First published on 29th May 2015
Abstract
In the search for fast lithium-ion conducting solids for the development of safe rechargeable all-solid-state batteries with high energy density, thiophosphates and related compounds have been demonstrated to be particularly promising both because of their record ionic conductivities and their typically low charge transfer resistances. In this work we explore a wide range of known and predicted thiophosphates with a particular focus on the cubic argyrodite phase with a robust three-dimensional network of ion migration pathways. Structural and hydrolysis stability are calculated employing density functional method in combination with a generally applicable method of predicting the relevant critical reaction. The activation energy for ion migration in these argyrodites is then calculated using the empirical bond valence pathway method developed in our group, while bandgaps of selected argyrodites are calculated as a basis for assessing the electrochemical window. Findings for the lithium compounds are also compared to those of previously known copper argyrodites and hypothetical sodium argyrodites. Therefrom, guidelines for experimental work are derived to yield phases with the optimum balance between chemical stability and ionic conductivity in the search for practical lithium and sodium solid electrolyte materials.
Introduction
Developing electrochemical storage devices with high energy densities is vitally important for powering our future society and the increasing demand for high energy and high-power rechargeable batteries has become the major driving force for further advances in battery technology. The available organic liquid electrolytes more and more are recognised as a bottle-neck in the effort to develop safe high-performance systems. Instead, inorganic fast ion-conducting solids1,2 with high electrochemical stability in contact with the anode (often Li metal) and cathode material, favourable mechanical properties, cost-efficient low temperature synthesis and sufficient kinetic stability for operation over a wide temperature window increasingly appear as key components in most of the promising next generation energy storage systems including both all-solid state batteries as well as (Li–air, Li–sulphur, Li-redox flow) battery concepts wherein electrode liquids or slurries are combined with solid electrolytes to facilitate scalability.High ionic conductivity is essential to achieve a high capacity, while low electronic conductivity mitigates the electron transport through the solid electrolyte, thus preventing self-discharge of the batteries. On the other hand a certain local concentration of mobile electrons at the electrode:electrolyte interfaces mitigates charge transfer resistances (which may become a challenge for some oxide solid electrolytes). Among the solid-state Li ion conductors developed so far, the most favourable characteristics for application in electrochemical cells in terms of record ionic conductivity and lowest charge transfer resistances have been achieved by lithium thiophosphates and related compounds such as Li7P3S11,3,4 LGPS,5,6 thio-Lisicon7 and a recent report on a “Li7P2S8I” of unknown structure.8 Therefore, it is critical to explore more in detail sulphide-based solid electrolytes to identify the most favourable compositions and structures for application as solid electrolytes in batteries. One of the ways to widen the range of known solid electrolytes with technologically interesting performance is to explore compounds containing more than one type of anions. Solid solutions of oxide- or sulphide-based solid electrolytes with alkali halides have in the past proven to be a successful strategy to enhance ionic conductivity in a wide range of Ag+, Cu+ or alkali based systems.2,9 This straightforwardly leads to the exploration of the fast-ion conducting halide-containing lithium argyrodites that are in the focus of this work.10
The argyrodite family of compounds with the general formula A12−m−x+(Mm+Y42−)Y2−x2−Xx− where A+ = Li+, Cu+, Ag+; Mm+ = Si4+, Ge4+, Sn4+, P5+, As5+; Y2− = O2−, S2−, Se2−, Te2−; X− = Cl−, Br−, I−; 0 ≤ x ≤ 2 are named after the mineral Ag8GeS6.11 Only a few argyrodites with trivalent M3+ = Fe3+, Al3+, Ga3+ have been reported.12 A wider range of silver and copper argyrodites had been described 70 years ago13 and subsequently studied more in depth by Kuhs et al.14 and Evain et al.15 Temperature-dependent order–disorder transitions and cationic disorder in the high-temperature phase yield moderately high ionic conductivity for the high-temperature polymorphs of quaternary Cu and Ag-containing argyrodites,14,16 while the corresponding halide-free ternary argyrodites are generally found to be semiconductors, for which changes in the fundamental absorption-edge related to the polymorphic transitions were evidenced.17 Lithium argyrodites first reported by the Deiseroth group10 are promising candidates for solid electrolytes. The most commonly studied argyrodite-type lithium ion conductor, Li6PS5Br, is reported to have a conductivity of 0.7−1 × 10−3 S cm−1.18,19 The wide electrochemical window and applicability of lithium argyrodites in various types of rechargeable all-solid batteries have been demonstrated earlier by our group.20,21 Still, as for most fast-ion conducting lithium thiophosphates, such as Li7P3S11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or LGPS,5,6 the structures of the known lithium argyrodites such as Li6PS5Br are only metastable limiting choices in their synthesis and raising questions about the long-term structural stability. More importantly, the lithium thiophosphates are typically unstable with respect to hydrolysis, so that they can only be handled in dry atmospheres, which is a serious cost factor for their technical application. Therefore this study aims at exploring the effect of possible changes in composition on the structural stability and hydrolysis stability.
3 or LGPS,5,6 the structures of the known lithium argyrodites such as Li6PS5Br are only metastable limiting choices in their synthesis and raising questions about the long-term structural stability. More importantly, the lithium thiophosphates are typically unstable with respect to hydrolysis, so that they can only be handled in dry atmospheres, which is a serious cost factor for their technical application. Therefore this study aims at exploring the effect of possible changes in composition on the structural stability and hydrolysis stability.
Most argyrodites adopt a cation-disordered high-temperature cubic “aristotype” phase in space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (see Fig. 1a) with a characteristic anion arrangement in interpenetrating icosahedral cages, but argyrodites may also crystallize in an intermediate state of disorder in the lower-symmetry cubic space group P213, as well as in cation-ordered low-temperature phases in orthorhombic space group Pmn21 (e.g. for Cu8GeS6 below 330 K22), Pna21 (e.g. for Ag8SiS6 below 507 K14) and/or a monoclinic structure in space group Cc (e.g. for Cu6PS5Br23).
3m (see Fig. 1a) with a characteristic anion arrangement in interpenetrating icosahedral cages, but argyrodites may also crystallize in an intermediate state of disorder in the lower-symmetry cubic space group P213, as well as in cation-ordered low-temperature phases in orthorhombic space group Pmn21 (e.g. for Cu8GeS6 below 330 K22), Pna21 (e.g. for Ag8SiS6 below 507 K14) and/or a monoclinic structure in space group Cc (e.g. for Cu6PS5Br23).
For the halide-rich range of the possible argyrodite stoichiometries the argyrodite structure type is in competition to a distinct orthorhombic structure in space group Pnma (see Fig. 1b): Ag6(GeS4)X2 (X = Cl, Br)24,25 as well as Ag6(SnS4)Br226 have been found experimentally as the first members of this family of “non-metallic filled NaHg2 phases”, i.e. the partial structure of the non-metal atoms S and X (Cl, Br) corresponds to the arrangement of Na and Hg atoms in NaHg2 (=X2S4) with Ge located in tetrahedral holes exclusively and Ag located in tetrahedral as well as octahedral holes.
Besides the required high ionic and low electronic structural conductivity, a solid electrolyte also needs to exhibit high hydrolysis stability. Structural stability affects how easily a material can be synthesized and how stable it is against degradation during cycling. Hydrolysis stability is especially important for alkali sulphide materials, most of which will rapidly degrade in contact with humid air (or aqueous catholytes). Therefore, a dry atmosphere is critically required throughout their preparation and battery assembly, increasing production costs and decreasing reliability of the batteries. When comparing compounds containing the same type of elements and hence the same potential decomposition products, the hydrolysis stability is obviously related to the structural stability of the compound.
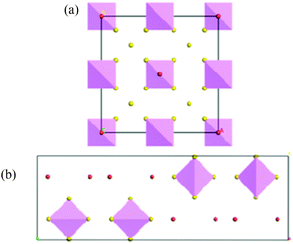 | ||
| Fig. 1 (a) Argyrodite structure and (b) NaHg2-related structure. Purple tetrahedrons represent MS48−m groups; red spheres represent X− anions; the mobile A+ ions are omitted. | ||
While structural stability, hydrolysis stability, ionic conductivity and electronic conductivity can in principle be determined experimentally, synthesis of some materials could be difficult, expensive or hazardous, so that computational approaches become beneficial to estimate these properties of materials, thus providing guidance to experimentalists whether synthesizing a new material appears worthwhile. Structural stability and hydrolysis stability can be assessed from the dissociation energy of the material. While the structural stabilities of various phases in the quaternary system Li–P–S–O are available in the literature,27 in this work we designed a systematic way to examine structural and hydrolysis stability of any phase of known stoichiometry based on the calculated formation energies. Predicting the ionic conductivity requires identifying the ion migration pathways of the mobile ions and their activation energies. We employed the empirical bond valence-based softBV method developed in our group.28–30 Electronic conductivity can be estimated from the determination of the bandgap by DFT calculations.
In this work, argyrodite-type and orthorhombic NaHg2-related phases of 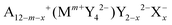 with A+ = Li+, Cu+, Ag+; Mm+ = Si4+, Ge4+, Sn4+, P5+, As5+; Y2− = O2−, S2−, Se2−, Te2−; X− = Cl−, Br−, I− are studied computationally to explore the suitability of this class of materials for practical applications in energy storage systems. Their structural stability and hydrolysis stability are determined by calculating the dissociation energies of the respective relevant critical reactions from ab initio data, and their ionic conductivities are assessed by empirical bond valence methods.
with A+ = Li+, Cu+, Ag+; Mm+ = Si4+, Ge4+, Sn4+, P5+, As5+; Y2− = O2−, S2−, Se2−, Te2−; X− = Cl−, Br−, I− are studied computationally to explore the suitability of this class of materials for practical applications in energy storage systems. Their structural stability and hydrolysis stability are determined by calculating the dissociation energies of the respective relevant critical reactions from ab initio data, and their ionic conductivities are assessed by empirical bond valence methods.
Depending on the halide content x (that may also adopt non-integer values), the cubic argyrodite phase may exhibit various degrees of site-inversion between anions X− and Y2−.10,18,19,21 Moreover, the effect of anion site-inversion on stability and conductivity on the argyrodites and related phases is examined in this study. To limit the computational effort, generally only stoichiometric compositions with integer values of x = 0, 1, 2 are considered.
Computational methodology
Structural stability
Structural stabilities are evaluated by the dissociation energy of the compound, which is the reaction energy of its critical reaction. Taking Li3PS4 for instance, its critical reaction is:| Li3PS4 ↔ ½S + ½Li2S + ½Li4P2S6 | (1) |
| ΔE = 0.5 × E(Li4P2S6) + 0.5 × E(Li2S) + 0.5 × E(S) − E(Li3PS4) | (2) |
Formation energy of compounds
The formation energy of a compound from its constituting elements is calculated by DFT methods as implemented in the VASP package31 based on a planewave basis set with using the Perdew–Burke–Ernzerhof (PBE) exchange–correlation parametrized general gradient approximation (GGA)32 and projector augmented-wave pseudopotentials (PAW).33,34 The cutoff threshold is set to 400 eV and convergence is by force < 0.03 eV Å−1. Each compound has a k-mesh with a density of at least 30 k-points × Å3. Although +U techniques help in more precisely evaluating energies of many transition metal oxides35 such as copper oxide, it is not applied here for two reasons: U values for most sulphides are not available in the literature; U values for oxides and sulphides are different, but in calculating hydrolysis stability, a single consistent U value would have to be used.Critical reaction
With the formation energy of a compound known, the respective critical reaction remains to be determined in order to calculate the dissociation energy. The critical reaction is the reaction with the most negative reaction energy among all conceivable dissociation reactions of the compound. While experimental observation and experience is helpful in identifying such critical reactions, it appears to be more consistent for the screening approach of this work here to follow a generally applicable and exhaustive approach.Consider a compound with formula A1b1A1b2…Ambm, where Ai are distinct elements, bi are the stoichiometric ratios, and i = 1, 2,…,m. Suppose there are n compounds in the phase diagram of A1–A2–…–Am, each is denoted by A1a1jA2a2j…Amamj, where j = 1, 2,…,n, and aij ≥ 0. Then all possible reaction equations can be summarized in the following form:
| A1b1A2b2…Ambm ↔ x1A1a11A2a21…Amam1 +…+ xnA1a1nA2a2n…Amamn | (3) |
Provided that the complete list of compounds in the phase diagram is known, the search for the critical reaction can be converted into a standard linear programming problem:
Minimize E = E·x = E1 × x1 + E2 × x2 +…+ En × xn, subject to the constraint A·x = b where:
A = {aij} are stoichiometric numbers of each element Ai in each of the conceivable decomposition products j;
b = {bi} are stoichiometric numbers of each element in the reference compound;
E = {Ei} are calculated formation energies of each compound;
x = {xi} are the coefficients of each compound to which the reference compound dissociates, x is the unknown to be solved and x ≥ 0;
This minimization task has a unique solution and is here solved by a simple program written with GLPK libraries36 that is available from the supplementary material.
Effects of anion site-inversion
In the aristotype argyrodite structures of Li11−m(Mm+Y4)YX, each unit cell contains four free Y2− anions that are not part of rigid (MY4)(8−m)− groups and four X− anions. To study the effect of anion site-inversion, the stabilities of five configurations are calculated for each composition by swapping zero to four pairs of Y2−/X− anions. The degree of site-inversion is defined to be 0% when all four free Y2− anions are located at the regular Y site, i.e. the 4d site‡ (¾, ¾, ¾), (¼, ¼, ¾), (¾, ¼, ¼), (¼, ¾, ¼), and four X− anions located at the 4a site (0, 0, 0), (½, ½, 1), (½, 1, ½), (1, ½, ½), which corresponds to the experimentally observed structure of Li6PS5I in space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m. By swapping one pair of Y2−/X− per unit cell, the degree of site-inversion is increased to 25%. To study the effect of site-inversion more in detail, a four-fold supercell has been constructed for the model compound Li6PS5Br, so that the model contains 16 pairs of swappable anions and the degree of site inversion can be modelled in steps of 6.25%.
3m. By swapping one pair of Y2−/X− per unit cell, the degree of site-inversion is increased to 25%. To study the effect of site-inversion more in detail, a four-fold supercell has been constructed for the model compound Li6PS5Br, so that the model contains 16 pairs of swappable anions and the degree of site inversion can be modelled in steps of 6.25%.
Hydrolysis stability
Hydrolysis stability of the lithium-containing sulphides is estimated by assuming the formation of the primary products LiOH and H2S. The calculation method is essentially the same as for evaluating structural stabilities. To identify the critical reaction of hydrolysis, it is assumed that an excess number n of water molecules is available and the resulting hydrolysis products contain oxides. In the system Li–P–S–H–O, the critical reaction is:| Li3PS4 + nH2O ↔ 4H2S + (n − 4)H2O + Li3PO4 | (4a) |
| Li3PS4 + 4H2O ↔ 4H2S + Li3PO4 | (4b) |
In practice, hydrolysis reactions may be more complex. The products may e.g. be amorphous and non-stoichiometric, and the actual reaction path may depend on a number of factors such as humidity, temperature, and reaction time. Thus, the results presented in this study can only provide a rough guideline for comparing the relative (thermodynamic) hydrolysis stability of different argyrodites.
Electronic conductivity
To compare the electronic conductivities of the argyrodite phases, the bandgaps of the structures are calculated by DFT calculations using VASP. Since the employed GGA functional has the intrinsic tendency to underestimate the bandgap, the resulting values have to be understood as lower bounds to the true bandgaps.Pathway for and activation energy of ionic conductivity
All cubic phases of argyrodites exhibit a three-dimensional network of pathways connecting partially occupied sites for the migration of the monovalent A+ ions and hence show ionic conductivity. In this report the activation energy for ion migration is estimated as the main influencing factor. Our empirical bond-valence based softBV forcefield37 is used to analyse the site energies of Li+ ions EBVSE(Li) throughout the structures of lithium argyrodites. Migration pathways for Li+ are then analysed as regions of low EBVSE(Li) in the resulting grids spanning the structure model with a resolution of typically (0.1 Å)3, which has been demonstrated to be a simple and reliable way of identifying transport pathways in local structure models,29,30 provided that the local structure model captures the essential structural features. For the purpose of analyzing EBVSE(Li) landscapes Coulomb repulsions are considered only between mobile and immobile cations (here e.g. Li+–P5+), while Coulomb attraction terms are generally integrated in the Morse attraction term. Starting from an analysis of local minima and saddle points of EBVSE(Li), the grid analysis utilizes a modified Dewar, Healy and Stewart (DHS)-type path finding algorithm38 to identify low energy paths connecting the local site energy minima.39Such energy landscapes based on rigid structure models without the possibility for structural relaxation inherently overestimate the energy barriers. In order to factor in the reduction of energy barriers by relaxation effects, the initially observed barriers needs to be scaled down by an empirical factor ≈0.5 that slightly depends on both the mobile ion and on the anion in its first coordination shell. For details on the scaling factor see Table S1 in the ESI.† By means of this scaling, the scaled predicted activation energies should become comparable to each other and to experimental results, while unscaled activation energies can only be compared among compounds with similar compositions.
Results and discussion
Substitution of Mm+ and X− in Li12−x−m(Mm+S4)S2−xXx
| Li12−x−mMm+S6−xXx ↔ Li8−mMm+S4 + (2 − x)Li2S + xLiX | (5) |
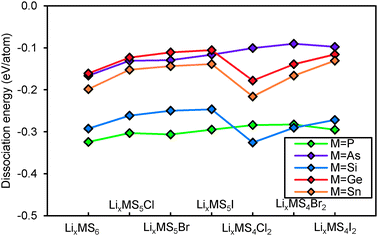
The results summarised in Fig. 2 show that most of the argyrodites are metastable, and thus may only be synthesized with suitable non-equilibrium low-temperature methods, as they might decompose at higher temperatures. With increasing halogen content, the structural stability of the NaHg2-related orthorhombic phases is enhanced when compared to the argyrodite phases of the same composition. In other words, the calculations show that for Li6MS4X2 phases with M4+ cations the NaHg2-related structures are more stable than the argyrodite phases (see Fig. 2a). For the M5+ cations the overall tendency is the same, but for the halide-richest compositions Li5MS4X2 the dissociation energies of argyrodite and NaHg2-phases are closely similar (see Fig. 2b).
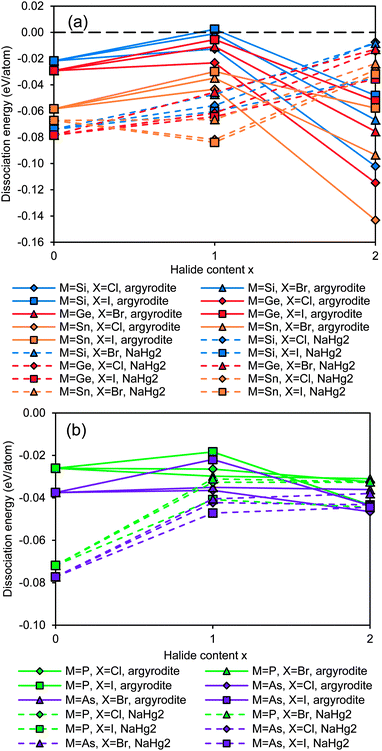 | ||
| Fig. 2 Structural stability of (a) Si,Ge,Sn-argyrodites and (b) P,As-argyrodites. Higher positions in the graph indicate higher stability. | ||
Compounds based on the heavier Mm+ cations As5+ and Sn4+ are overall less stable than those with the lighter Mm+ cations and the least stable compounds involve these heavy M+ cations in combination with the lightest halide X− = Cl−, so that the thermal stability will be further reduced and it may become more difficult to synthesize and stabilize them. In line with this, it was e.g. found that while argyrodites with M = P can be formed for X = Cl, Br or I, for M = As only those with X = Br and I can be synthesized.40 In terms of choices of halogen elements, argyrodite phases containing iodide are more stable than those containing bromide and chloride.
When considering the dependence of the structural stability of the Li12−x−m(Mm+S4)S2−xXx argyrodites on the halide content x, the dissociation energies shown in Fig. 2 as a function of halide content x exhibit a clear maximum at x = 1 for M4+ compounds (cf. solid lines in Fig. 2a), while among the M5+ compounds only those with X− = I− show a comparatively pronounced stability maximum. Therefrom it may be concluded that for these two groups of argyrodites a (near) stoichiometric composition with x = 1 will be preferred (as also found experimentally for Li6M5+S5I argyrodites).10,19,40 For the M5+ argyrodites with the lighter halide anions Br− and Cl− the dissociation energy decreases slightly as x increases from 0 to 1 (for the case of M = P) or is practically constant (for M = As). In these compounds the entropy influence may preferentially lead to non-stoichiometric compounds with compositions controlled by details of the sample preparation conditions. Such a behaviour is experimentally observed for Li7−xMS6−xXx (with M = P, X = Cl or Br and to some extent for M = As, X = Br, where only a slight deviation from stoichiometry is noted).10,21,40
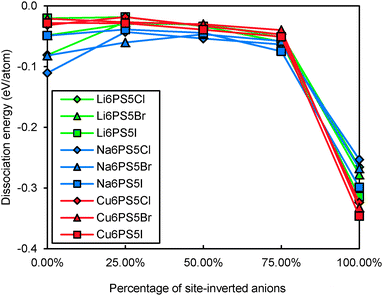
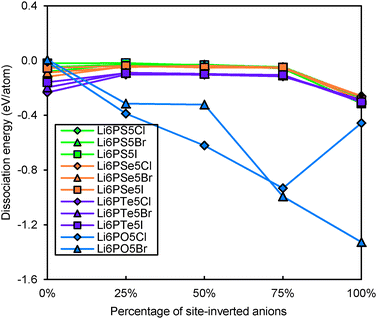
Even for stoichiometric argyrodites Li11−mMS5X (which are, as mentioned above, often close to the most preferable composition) the influence of disorder may be relevant for their structural stability. From earlier structural studies, it is known that e.g. in Li7−xPS6−xXx with X = Cl, Br the experimentally observed S2−/X− anion distribution is disordered in the sense that there is a partial inversion between the occupancies of the nominal S2− and X− sites. Detailed neutron diffraction studies indicate a temperature dependence of this disorder.21 Here we studied whether there is a thermodynamic driving force for the site inversion. Fig. 3 surveys the effect of site-inversion on the dissociation energies of the studied argyrodites with x = 1. Swapping the occupancies of the four pairs of S2−/X− anions per unit cell allows varying the site occupancies in steps of 25%. From the results in Fig. 3 the effect of the S2−/X− site-inversion on the structural stability seems to depend on the oxidation state m of the Mm+ cation and the type of halogen atom involved. In argyrodites containing M5+ cations, chlorides and bromides prefer around 25% site-inversion while iodides prefer the fully ordered structure (0% site-inversion). This suggests that the experimentally observed site-inversion for Li6PS5Cl and Li6PS5Br (and fully ordered nature of the anion distribution in Li6PS5I) is caused by an enthalpic driving force and not only a kinetic effect.
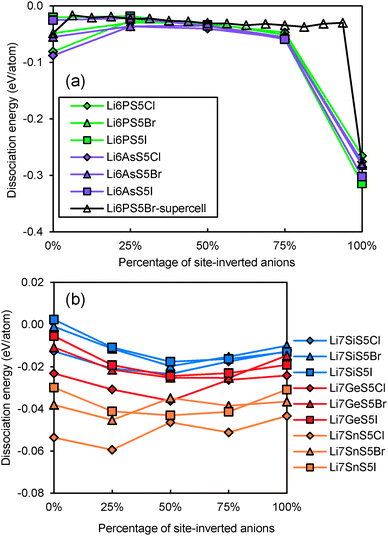 | ||
| Fig. 3 Variation of dissociation energies of (a) P,As-argyrodites and (b) Si,Ge,Sn-argyrodites with the degree of S2−/X− anion site-inversion. | ||
To understand more in detail the correlation between anion site-inversion and stability, configurations with random pairs of S2−/Br− swapped have also been calculated for a four-fold supercell in the case of Li6PS5Br. Results are shown as open triangles in Fig. 3(a). Both fully ordered structures (no inversion or complete inversion) exhibit significantly more negative dissociation energy than the structures with partial S2−/Br− swapping. The dissociation energy reaches its least negative value for low degrees of site inversion (6–19%) but show only a minute decrease towards higher degrees of site inversion before suddenly shooting down to the most negative value for the fully-inverted structure. The energies found for the same degrees of site inversion for the single cell and four-fold supercells of Li6PS5Br reasonably agree with each other, so that the fundamental variations for the other compounds may be estimated from the computationally less demanding single unit cell calculations in Fig. 3. In argyrodites containing M4+ cations (see Fig. 3(b)), the influence of site-inversion on structural stability is mostly reversed. Structures with 0% and 100% site-inversion are typically similar in dissociation energy and more stable than structures with 25–75% site-inversion. As mentioned above the compounds with M = Sn show the lowest stability independent of the site inversion and a less regular variation of the dissociation energy with the degree of anion site inversion.
| Li7−xPS6−xXx + (8 − 2x)H2O ↔ xLiX + Li3PO4 + (6 − x)H2S + (4 − 2x)LiOH | (6) |
| Li7−xAsS6−xXx + (7 − 2x)H2O ↔ xLiX + 0.5As2S3 + S + (3.5 − x)H2S + (7 − 2x)LiOH | (7) |
| Li8−xSiS6−xXx + (9 − 2x)H2O ↔ xLiX + Li2SiO3 + (6 − x)H2S + (6 − 2x)LiOH | (8) |
| Li8−xGeS6−xXx + (8 − 2x)H2O ↔ xLiX + GeS2 + (4 − x)H2S + (8 − 2x)LiOH | (9) |
| Li8−xSnS6−xXx + (8 − 2x)H2O ↔ xLiX + SnS2 + (4−x)H2S + (8 − 2x)LiOH | (10) |
When comparing the resulting hydrolysis stability values for Li12−x−mMm+S6−xXx argyrodites in Fig. 4 it becomes obvious that all these lithium argyrodites will decompose in contact with water, as indicated by the negative dissociation energies. Phosphorus and silicon argyrodites are highly sensitive to moisture (with the commonly studied P-compounds typically being the worst case in terms of hydrolysis stability), while germanium, tin and arsenic argyrodites are significantly less vulnerable to hydrolysis. It should however be kept in mind that the latter two also have a lower structural stability. In terms of the halide content, compounds without any halogen atoms demonstrate lower hydrolysis stability, and the vulnerability to hydrolysis only slightly decreases with the increase of the size of the halogen atom among the Li11−mMm+S5X argyrodites with the same halide content.
Li+ migration in argyrodites Li12−mMm+S6 and Li11−mMm+S5X requires lower activation energies than in halide-rich argyrodites Li10−mMm+S4X2. Moreover argyrodites containing I− tend to have higher activation energies than those containing Cl− and Br− in line with a more general rough trend that the more stable (anion ordered) compounds tend to show somewhat higher activation energies also for the Li+ mobility. In addition, the lower repulsion of M4+ and higher concentration of mobile charge carriers seems to favour on average somewhat lower activation energies in Li7M4+S5X than in Li6M5+S5X compounds. Moreover, NaHg2-related Li7M4+S5X phases are found to have higher (and the Li6M4+S5X2 compounds lower) activation energies than the argyrodites of the same composition, while for the phases containing M5+ no such systematic difference between the two structure types can be observed in terms of their activation energy for Li+ migration. The influence of the S2−/Br− anion site-inversion in local supercell structure models of the Li6PS5Br on the predicted activation energy barrier for Li+ is demonstrated in Fig. 6. Configurations of 100%, 50% and 0% site-inversion are found to have the smallest activation energies. The specific choice of local structures may affect this finding. Still, the observation that the difference in activation energy between the highest and lowest activation energy configurations for the same composition reaches about 0.20 eV suggests that anion disorder in argyrodites strongly influences the local and overall lithium ion conductivity.
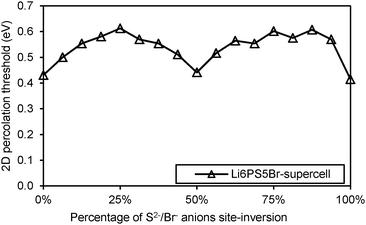 | ||
| Fig. 6 Activation energy required for Li+ migration in site-inverted configurations of Li6PS5Br supercell. | ||
To assess the accuracy of the empirical approach for estimating migration barriers from structure models using the softBV forcefield, experimental activation energy data from the literature conductivity and NMR studies are compared to the calculated migration barriers.16,41–45 While local structures generated from DFT geometry-optimisation do not readily accommodate fractional occupancy and show some deviation in lattice parameters compared to experimentally determined structures, these comparisons are based on experimental structure data.,10,40,46 Results are summarised in Fig. 7. It should be noted that among the calculated barrier energies values <0.2 eV refer to barriers for local hopping and the corresponding experimental data are NMR data, while values >0.2 eV correspond to the migration barrier for percolating pathways. As seen from Fig. 7 the limiting factor for the quality of the correlation is less the quality of the computational prediction but the large spread in the experimental activation energies reported in the literature for the same compound.
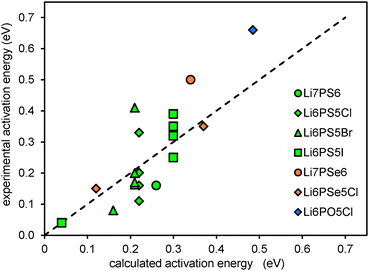 | ||
| Fig. 7 Comparison of experimental activation energies to calculated activation energies based on experimentally determined structures. | ||
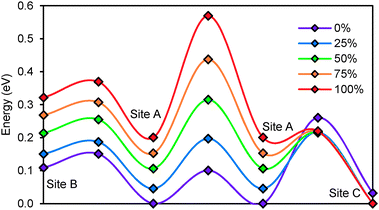
Li+ ions in argyrodites are generally found to be distributed over three types of sites. Site A (48 h) refers to the equilibrium site that hosts the bulk of Li+ ions in all argyrodite structures. Interstitial sites of type B (48 h) are located in-between A sites, so that 3A and 3B sites nearly form a low energy pathway hexagon. In combination with local paths connecting directly two A sites 4 such A–B hexagons form a local pathway cage around each S2− anion in Li6PS5I. The A sites in these cages are then connected via type C interstitial sites (16e) to a 3-dimensional migration pathway network. In Li6PSe5Cl the local (A–B)3 pathway hexagons are bridged by A–C–A pathways to local pathway cages around the halide ion that only for higher activation energies merge to a percolating pathway network by direct A–A bridges.
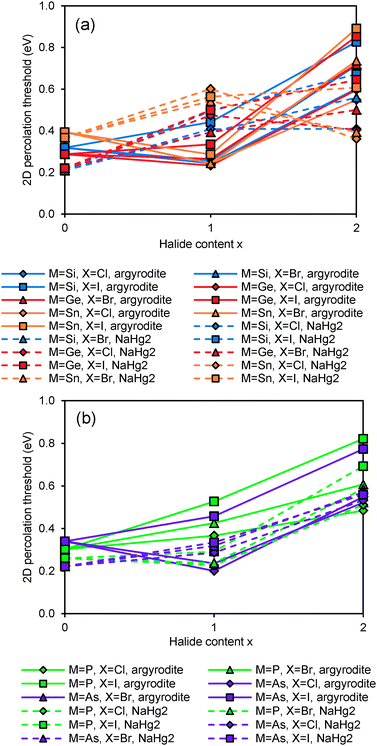
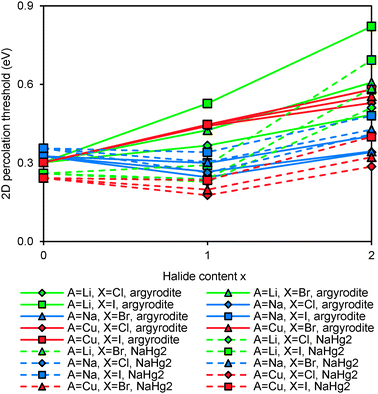
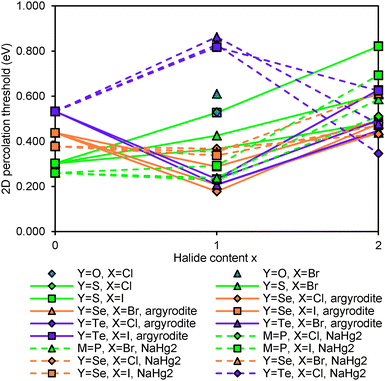
The relative site energies and activation barriers between sites are summarised in Fig. 8, in which the lowest site energy among the three types of sites is set to 0 eV. The percolation threshold (or the long-range activation energy) equals to the maximum of the three relevant migration barriers, i.e. either the barrier between two A sites or between an A site and a C site. It may also be noted that their respective barrier heights are anticorrelated. Li6PS5I, for example, shows a very low activation energy between two A sites which may correspond to the experimentally observed short-range activation energy of 0.04 eV, but the much larger activation energy between A and C sites causes the relatively high activation energy of about 0.35 eV for dc conductivity.44 When the size of the halide ion is reduced by substituting I− by Br− the A–C barrier shrinks and the A–A barrier increases so that the activation energy for local motions slightly increases, while the activation energy for dc conductivity is considerably reduced.
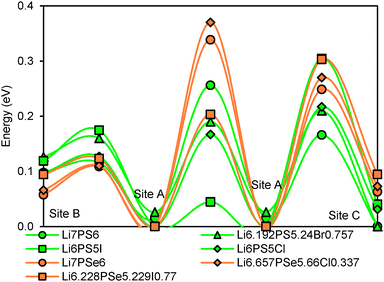 | ||
| Fig. 8 Site energies and activation barriers between neighbouring sites in sulphide and selenide argyrodites. | ||
To study the effect of anion disorder on the migration barriers between the different sites, we analysed the barriers in hypothetical structures derived from the experimental structure of Li6PS5Br. A fully stoichiometric and anion ordered structure is compared to structures with mixed anion site occupancy, where in steps of 0.25 increasing fractions of Br− are assigned to the 4d sites, while the corresponding amount of S2− is redistributed to the 4a sites. The effect of the different degrees of anion disorder on the migrations barriers in Li6PS5Br are summarized in Fig. 9. The increase in anion disorder has little effect on the height of the migration barrier between A and B sites, but consistently increases barrier heights between two A sites and decreases activation energies between A and C sites. For the case of Li6PS5Br the lowest activation energy for long-range ion migration (dc conductivity) results for 25% anion disorder, when the barrier heights for A–A and A–C hops are almost equal.
Substitution of A+ and X− in A7−x(PS4)S2−xXx argyrodites
Critical reactions for structural stability are given in eqn (5), (11) and (12) for A = Li, Na or Cu, respectively:
| Na7−xPS6−xXx ↔ xNaX + (2 − x)Na2S + Na3PS4 | (11) |
| Cu7−xPS6−xXx ↔ xCuX + (0.5 − 0.25x)Cu + (0.5 − 0.25x)Cu7S4 + Cu3PS4 | (12) |
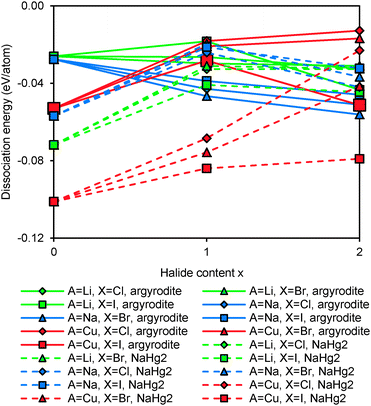
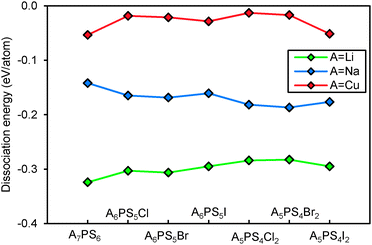
Fig. 10 compares the structural stability of compounds containing different mobile cations A+. Sodium argyrodites are found to be less stable than lithium and copper argyrodites, consistent with the fact that most of the discovered argyrodites contain copper, silver and lithium instead of sodium. From Fig. 10, Na7PS6 may be the only practically accessible sodium argyrodite phase. Among the studied phases copper argyrodites are found to be the most stable, in line with the existence of natural copper-containing argyrodite Ag3.48Cu4.68GeS6 in minerals.47 Copper argyrodites are always more stable than the NaHg2-related phases of the same composition. Similar to Li6PS5I and in line with experimental structure determinations, Cu6PS5I is expected to be stoichiometric from the pronounced stability maximum around x = 1.48,49
Again we explored possible effects of anion site site-inversion on stability of A-argyrodites and the findings are summarized in Fig. 11. The overall shapes of the stability vs. degree of site inversion curves are similar for all three types of compounds: with the increase in size of halogen anions (and thus in the X− sulphide size difference), the most stable degree of site-inversion shifts from 25% towards 0%. Argyrodites containing I− always prefer the fully ordered configuration. This suggests that the nature of the A+ ion has only a minor influence on the preferable degree of site inversion.
| Na7−xPS6−xX + (8 − 2x)H2O ↔ xNaX + Na3PO4 + (6 − x)H2S + (4 − 2x)NaOH | (13) |
| Cu7−xPS6−xXx + 5/32(2 − x)H2O ↔ xCuX + 1/32(2 − x)P2O5 + 1/16(14 + x)Cu3PS4 + 5/16(2 − x)Cu7S4 + 5/32(2 − x)H2 | (14) |
Fig. 12 shows the relative stability against moisture of compounds containing the three types of mobile A+ cations. Argyrodites containing copper are much more stable to moisture than the alkali argyrodites. This is because copper sulphide (Cu7S4) and copper thiophosphate (Cu3PS4) are more stable than their lithium and sodium counterparts in water. The predicted decomposition reaction of copper argyrodites would thus release H2 rather than H2S (as released by Li and Na argyrodites) and the released amount is much smaller compared to that of H2S in the case of the alkali. The higher hydrolysis stability of sodium argyrodites compared to lithium argyrodites is less intuitive. It may be affected by our approach of limiting ourselves to the primary reaction products AOH and hydrogen-free A oxides. Both of them will further react with water and the reaction energy depends largely on element A.
Substitution of Y2− and X− in Li7−x(PY4)Y2−xXx argyrodites
Critical reactions for structural stability are determined as eqn (5) and (15)–(19):
| Li6PO5X ↔ LiCl + Li2O + Li3PO4 | (15) |
| Li7PSe6 ↔ 2Li2Se + Li3PSe4 | (16) |
| Li6PSe5X ↔ 0.5Se + 1.5Li2Se + LiX + 0.5Li4P2Se6 | (17) |
| Li5PSe4X2 ↔ 0.5Se + 0.5Li2Se + 2LiX + 0.5Li4P2Se6 | (18) |
| Li7−xPTe6−xXx ↔ xLiX + P + 2.5Te + (3.5 − x)Li2Te | (19) |
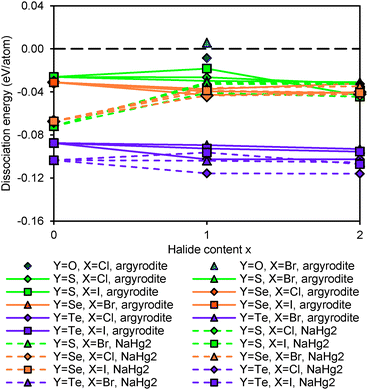
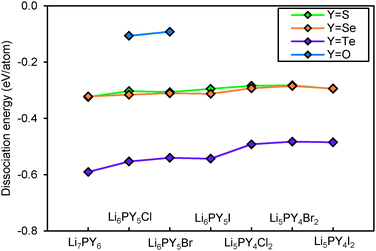
Fig. 14 shows the dissociation energy of compounds containing different Y2− anions. The structural stability of lithium argyrodites containing Y2− increases in the order of Te < Se < S < O. The tellurium compounds show considerably more negative dissociation energies indicating that they may practically not be accessible. Selenium compounds, on the other hand, have similar dissociation energies to those of sulphur compounds. Except for Li7PSe6 which has been successfully synthesized in the argyrodite structure,46 the likelihood for selenium compounds containing halogen elements to crystallize in argyrodite phase and NaHg2-related phase are almost equal. It should be stressed that the critical reaction of selenium argyrodites takes only three Li–P–Se ternary compounds into consideration. Two of them are Li4P2Se650 and Li7PSe6 as reported in a study by Francisco.51 Another compound Li4PSe3 is constructed by replacing sulphur atoms in Li3PS4 with selenium atoms. Li4P2Se6 is found to be the most stable ternary compound calculated so far. With the addition of new compounds into the phase Li–P–Se diagram, the critical reaction may change and the dissociation energy may become more negative. For all the different chalcogenide compounds studied the argyrodite phases are significantly preferred over NaHg2-related phases in the absence of halide anions, while for the halide-containing compounds the stability differences between the compounds with the two structure types are much smaller.
Fig. 15 plots the dissociation energy versus degree of site-inversion in Y-argyrodites. Selenium and tellurium argyrodites share the same trend as sulphur argyrodites that fully ordered configurations are less stable. In contrast the oxygen argyrodites can only exist in the fully ordered configuration. This is consistent with the experimental structure determination of oxygen argyrodites.45
| Li6PO5X + H2O ↔ LiX + Li3PO4 + 2LiOH | (20) |
| Li6PSe5X + (8 − 2x)H2O ↔ xLiX + Li3PO4 + (6 − x)Se + (6 − x)H2 + (4 − 2x)LiOH | (21) |
| Li6PTe5X + (8 − 2x)H2O ↔ xLiX + Li3PO4 + (6 − x)Te + (6 − x)H2 + (4 − 2x)LiOH | (22) |
Fig. 16 shows the resulting hydrolysis dissociation energies of argyrodites. Oxygen argyrodites have the highest calculated hydrolysis stability, in line with the general tendency that oxides are more stable to moisture than sulphides, selenides and tellurides. Similar to the findings for structural stability, selenium argyrodites and sulphur argyrodites have nearly the same level of hydrolysis stability. The hydrolysis products are different, with selenium argyrodites releasing H2 while sulphur argyrodites releasing H2S. Though H2Se and H2Te exist in gaseous form, they are not stable and would decompose into H2 and Se/Te.
| Argyrodite cubic phase | Calculated bandgap (eV) |
|---|---|
| Li6PO5Br | 5.67 |
| Li6PS5Br | 2.12 |
| Li6PSe5Br | 1.00 |
| Li6PTe5Br | 0.00 |
Based on these calculations the stability and electrochemical window of argyrodites should increase in the order of Te ≪ Se < S < O. While selenium argyrodites might be accessible, their limited stability will probably remain an issue for practical applications. Among the four classes of argyrodites, sulphur argyrodites still strike the best balance between stability and conductivity.
Concluding remarks
The search for solid electrolyte materials is often a compromise between chemical stability and ionic conductivity of a compound. Using argyrodite systems as an example, a systematic evaluation of structural stability, hydrolysis stability, ionic conductivity and electronic conductivity is achieved combining various computational techniques. The results show that there is significant room for synthesizing argyrodite compounds with improved stability and conductivity as solid electrolyte materials for ion-conducting rechargeable batteries.Acknowledgements
This research is supported by the National Research Foundation, Prime Minister's office, Singapore under its Competitive Research Programme (CRP Awards No. NRF-CRP 10-2012-6 and NRF-CRP 8-2011-04).References
- M. Tatsumisago, M. Nagao and A. Hayashi, J. Australas. Ceram. Soc., 2013, 1, 17–25 CrossRef PubMed.
- P. Knauth, Solid State Ionics, 2009, 180, 911–916 CrossRef CAS PubMed.
- H. Yamane, M. Shibata, Y. Shimane, T. Junke, Y. Seino, S. Adams, K. Minami, A. Hayashi and M. Tatsumisago, Solid State Ionics, 2007, 178, 1163–1167 CrossRef CAS PubMed.
- Y. Seino, T. Ota, K. Takada, A. Hayashi and M. Tatsumisago, Energy Environ. Sci., 2014, 7, 627–631 CAS.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Matsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- S. Adams and R. P. Rao, J. Mater. Chem., 2012, 22, 7687–7691 RSC.
- R. Kanno, T. Hata, Y. Kawamoto and M. Irie, Solid State Ionics, 2000, 130, 97–104 CrossRef CAS.
- E. Rangasamy, Z. Liu, M. Gobet, K. Pilar, G. Sahu, W. Zhou, H. Wu, S. Greenbaum and C. Liang, J. Am. Chem. Soc., 2015, 137, 1384–1387 CrossRef CAS PubMed.
- S. Adams and J. Swenson, Phys. Chem. Chem. Phys., 2002, 4, 3179–3184 RSC.
- H.-J. Deiseroth, S.-T. Kong, H. Eckert, J. Vannahme, C. Reiner, T. Zaiß and M. Schlosser, Angew. Chem., Int. Ed., 2008, 47, 755–758 CrossRef CAS PubMed.
- C. Winkler, Ber. Bunsen-Ges., 1886, 19, 210–211 Search PubMed.
- D. Frank, B. Gerke, M. Eul, R. Pöttgen and A. Pfitzner, Chem. Mater., 2013, 25, 2339–2345 CrossRef CAS.
- C. Palache, H. Berman and C. Frondel, Dana's system of mineralogy, John Wiley & Son's, New York, 7th edn, 1944, vol. 1, pp. 356–358 Search PubMed.
- (a) W. F. Kuhs, R. Nitsche and K. Scheunemann, Mater. Res. Bull., 1979, 14, 241–248 CrossRef CAS; (b) W. F. Kuhs and G. Heger, in Fast ion transport in solids: electrodes, and electrolytes, ed. P. Vashishta, J. N. Mundy and G. K. Shenoyeds, Proceedings of the International Conference on Fast Ion Transport in Solids, Electrodes, and Electrolytes, Lake Geneva, Wisconsin, USA, May 21–25, Elsevier, Amsterdam, 1979, pp. 233–236 Search PubMed.
- (a) M. Evain, E. Gaudin, F. Boucher, V. Petricek and F. Taulelle, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 1998, 54, 376–383 CrossRef; (b) E. Gaudin, F. Boucher, V. Petricek, F. Taulelle and M. Evain, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2000, 56, 402–408 Search PubMed; (c) E. Gaudin, V. Petricek, F. Boucher, F. Taulelle and M. Evain, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2000, 56, 972–979 CrossRef PubMed.
- (a) H. Wada, J. Alloys Compd., 1992, 178, 315–323 CrossRef CAS; (b) H. Wada, A. Sato, M. Onoda, S. Adams, M. Tansho and M. Ishii, Solid State Ionics, 2002, 154–155, 723–727 CrossRef CAS.
- A. S. Kinduris, R. A. Bendoryus and D. B. Senulene, Sov. Phys. Semicond., 1976, 10, 916–917 Search PubMed.
- (a) R. P. Rao and S. Adams, Phys. Status Solidi A, 2011, 208, 1804–1807 CrossRef CAS PubMed; (b) S. Adams and R. Prasada Rao, Understanding Ionic Conduction and Energy Storage Materials with Bond-Valence-Based Methods, in Bond Valences, Structure and Bonding, ed. I. D. Brown and K. R. Poeppelmeier, Springer, Berlin, Heidelberg, 2014, vol. 158, pp. 129–159 Search PubMed.
- R. P. Rao, N. Sharma, V. K. Peterson and S. Adams, J. Solid State Electrochem., 2012, 16, 1807–1813 CrossRef.
- M. Chen, R. P. Rao and S. Adams, Solid State Ionics, 2014, 262, 183–187 CrossRef CAS PubMed.
- R. P. Rao, N. Sharma, V. K. Peterson and S. Adams, Solid State Ionics, 2013, 230, 72–76 CrossRef CAS PubMed.
- M. Ishii, M. Onoda and K. Shibata, Solid State Ionics, 1999, 121, 11–18 CrossRef CAS.
- A. Haznar, A. Pietraszko and I. P. Studenyak, Solid State Ionics, 1999, 119, 31–36 CrossRef CAS.
- (a) M. Wagener, H.-J. Deiseroth and C. Reiner, Z. Kristallogr., 2006, 221, 533–538 CrossRef CAS PubMed; (b) M. Wagener, PhD thesis, University of Siegen, 2005.
- M. V. Moroz, P. Y. Demchenko, L. G. Akselrud, O. G. Mykolaychuk and R. E. Gladyshevskii, Chem. Met. Alloys, 2010, 3, 161–168 Search PubMed.
- O. G. Mykolaychuk, N. V. Moroz, P. Yu. Demchenko, L. G. Akselrud and R. E. Gladyshevskii, Inorg. Mater., 2010, 46, 590–597 CrossRef.
- N. A. W. Holzwarth, N. D. Lepley and Y. A. Du, J. Power Sources, 2011, 196, 6870–6876 CrossRef CAS PubMed.
- S. Adams, Solid State Ionics, 2000, 136, 1351–1361 CrossRef.
- (a) S. Adams and R. P. Rao, Phys. Status Solidi A, 2011, 208, 1746–1753 CrossRef CAS PubMed; (b) S. Adams and R. P. Rao, in Bond Valences, ed. I. D. Brown and K. R. Poeppelmeier, Springer-Verlag, Berlin Heidelberg, 2014, ch. 5, pp. 129–159 Search PubMed.
- S. Adams and R. P. Rao, Solid State Ionics, 2011, 184, 57–61 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- G. Kresse and J. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- L. Wang, T. Maxisch and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 195107 CrossRef.
- GNU Linear Programming Kit, version 4.55, http://www.gnu.org/software/glpk/glpk.html.
- S. Adams, Practical Considerations in Determining Bond Valence Parameters, in Bond Valences, Structure and Bonding, ed. I. D. Brown and K. R. Poeppelmeier, Springer, Berlin, Heidelberg, 2014, vol. 158, pp. 91–128 Search PubMed.
- L. L. Wong, H. M. Chen and S. Adams, Phys. Chem. Chem. Phys., 2015, 17, 9186–9193 RSC.
- M. J. S. Dewar, E. F. Healy and J. J. P. Stewart, J. Chem. Soc., Faraday Trans. 2, 1984, 280, 227–233 RSC.
- S.-T. Kong, H.-J. Deiseroth, C. Reiner, Ö. Gün, E. Neumann, C. Ritter and D. Zahn, Chem. – Eur. J., 2010, 16, 2198–2206 CrossRef CAS PubMed.
- H.-J. Deiseroth, J. Maier, K. Weichert, V. Nickel, S.-T. Kong and C. Reiner, Z. Anorg. Allg. Chem., 2011, 637, 1287–1294 CrossRef CAS PubMed.
- S. Boulineau, M. Courty, J.-M. Tarascon and V. Viallet, Solid State Ionics, 2012, 221, 1–5 CrossRef CAS PubMed.
- V. Epp, Ö. Gün, H.-J. Deiseroth and M. Wilkening, J. Phys. Chem. Lett., 2013, 4, 2118–2123 CrossRef CAS.
- O. Pecher, S.-T. Kong, T. Goebel, V. Nickel, K. Weichert, C. Reiner, H.-J. Deiseroth, J. Maier, F. Haarmann and D. Zahn, Chem. – Eur. J., 2010, 16, 8347–8354 CrossRef CAS PubMed.
- S.-T. Kong, H.-J. Deiseroth, J. Maier, V. Nickel, K. Weichert and C. Reiner, Z. Anorg. Allg. Chem., 2010, 636, 1920–1924 CrossRef CAS PubMed.
- S.-T. Kong, O. Gün, B. Koch, H.-J. Deiseroth, H. Eckert and C. Reiner, Chem. – Eur. J., 2010, 16, 5138–5147 CrossRef CAS PubMed.
- W. H. Paar, A. C. Roberts, P. Berlepsch, T. Armbruster, D. Topa and G. Zagler, Can. Mineral., 2004, 42, 1757–1769 CrossRef CAS.
- A. Ggor, A. Pietraszko and D. Kaynts, J. Solid State Chem., 2005, 178, 3366–3375 CrossRef PubMed.
- T. Nilges and A. Pfitzner, Z. Kristallogr., 2005, 220, 281–294 CrossRef CAS.
- Q. Shi, Master thesis, Shandong University, 2012.
- R. H. P. Francisco, J. Solid State Chem., 1993, 107, 452–459 CrossRef CAS.
- V. Epp, Ö. Gün, H.-J. Deiseroth and M. Wilkening, Phys. Chem. Chem. Phys., 2013, 15, 7123–7132 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Program to evaluate critical reactions; tabulated values used to plot graphs. See DOI: 10.1039/c5cp01841b |
‡ The Wyckoff site names 4d and 4a strictly speaking apply only to the most common case of space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m. For the lower symmetry cubic Argyrodites both sites would be of type 4a, but still symmetry-independent. 3m. For the lower symmetry cubic Argyrodites both sites would be of type 4a, but still symmetry-independent. |
| This journal is © the Owner Societies 2015 |