Size-dependent electronic structure controls activity for ethanol electro-oxidation at Ptn/indium tin oxide (n = 1 to 14)†
Abstract
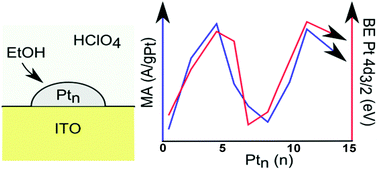
Understanding the factors that control electrochemical catalysis is essential to improving performance. We report a study of electrocatalytic ethanol oxidation – a process important for direct ethanol fuel cells – over size-selected Pt centers ranging from single atoms to Pt14. Model electrodes were prepared by soft-landing of mass-selected Ptn+ on indium tin oxide (ITO) supports in ultrahigh vacuum, and transferred to an in situ electrochemical cell without exposure to air. Each electrode had identical Pt coverage, and differed only in the size of Pt clusters deposited. The small Ptn have activities that vary strongly, and non-monotonically with deposited size. Activity per gram Pt ranges up to ten times higher than that of 5 to 10 nm Pt particles dispersed on ITO. Activity is anti-correlated with the Pt 4d core orbital binding energy, indicating that electron rich clusters are essential for high activity.

 Please wait while we load your content...
Please wait while we load your content...