Combining gas phase electron capture and IRMPD action spectroscopy to probe the electronic structure of a metastable reduced organometallic complex containing a non-innocent ligand†
Abstract
Combining electron capture dissociation mass spectrometry and infrared multiple photon dissociation action spectroscopy allows the formation, selection and characterisation of reduced metal complexes containing non-innocent ligands. Zinc complexes containing diazafluorenone ligands have been studied and the localisation of the single electron on the metal atom in the mono–ligated complex has been demonstrated.
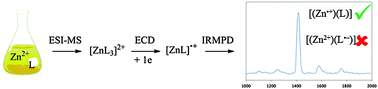
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...