Optical properties of prodigiosin and obatoclax: action spectroscopy and theoretical calculations†
Abstract
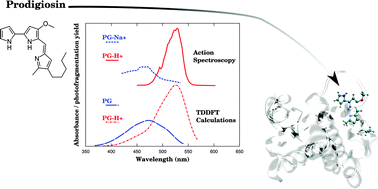
Prodiginine molecules (prodigiosin and obatoclax) are well-known pH-chromic dyes with promising anti-tumor properties. They present multiple tautomeric and rotameric forms. The protonation state and the structure of such flexible ligands in interaction with a protein are crucial to understand and to model the protein's biological activities. The determination of the protonation state via UV/vis absorption is possible if the ligand spectra of the neutral and protonated states are sufficiently different, and also if we can eliminate other factors potentially impacting the spectrum. Upon measuring the absorption spectra of the ligand in solution, varying solvents and pH values, we have determined that the optical properties of prodigiosin and obatoclax depend on the protonation state and not on the solvent permittivity constant. In parallel, action spectroscopy (using tunable lasers coupled to ion traps) in the gas phase of protonated and sodiated prodigiosin and obatoclax molecules has been performed to evaluate the sensitivity of the charge and the conformational state to their optical properties free of solvent. The spectra are interpreted using computational simulations of molecular structures and electronic excitations. The excitation energies are only slightly sensitive to various isomerizations, and may be used to distinguish between protonated and deprotonated states, even in the presence of a sodium counter-ion.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...