An infrared spectroscopic and theoretical study on (CH3)3N–H+–(H2O)n, n = 1–22: highly polarized hydrogen bond networks of hydrated clusters†
Abstract
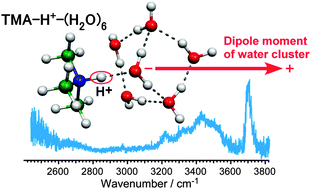
Infrared spectra of protonated trimethylamine (TMA)–water clusters, (CH3)3N–H+–(H2O)n (n = 1–22) were measured in the OH stretching vibrational region by size-selective photodissociation spectroscopy. Density functional theory calculations of stable structures were performed, and temperature dependence of the isomer populations and infrared spectra was also simulated by the harmonic superposition approximation approach to analyze hydrogen bond network structures in the clusters. It was shown that the excess proton (H+) in this system localizes on the TMA moiety regardless of cluster size. In the small-sized clusters, many isomers coexist and their hydrogen bond networks are highly polarized to induce the large charge-dipole interaction to stabilize the excess proton. Magic number behavior is not observed at around the magic number size (n = 21) of protonated water clusters and its implication on the hydrogen bond network structures is discussed.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...