Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning of electron transfer reactions in pluronic–surfactant supramolecular assemblies
Poonam
Verma
a and
Haridas
Pal
*b
aRadioanalytical Chemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400 085, India. E-mail: poonamv@barc.gov.in
bRadiation & Photochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400 085, India. E-mail: hpal@barc.gov.in
First published on 18th May 2015
Abstract
Photoinduced electron transfer (ET) reaction between an anionic acceptor, coumarin-343 (C343), and a neutral donor, N,N-dimethylaniline (DMAN), has been investigated in composite supramolecular assemblies (mixed micelles) comprised of a pluronic copolymer (P123: EO20–PO70–EO20 or F88: EO103–PO39–EO103 where EO: ethylene oxide and PO: propylene oxide) and a cationic surfactant (CTAC: cetyltrimethylammonium chloride), following fluorescence quenching studies. Systematic increase in the quenching rates for the studied donor–acceptor system with the increasing CTAC to pluronic molar ratio in the mixed micelles demonstrates a large modulation in the ET rates. The mixed micellar systems in the present cases are formed through the incorporation of the hydrocarbon chains of CTAC into the poly-PO core of the pluronic micelles whereby the cationic head groups of CTAC are placed at the periphery of the micellar core, protruded into the hydrated poly-EO corona region, leading to the formation of a positively charged layer deep inside these mixed micelles. Thus, the anionic C343 dye, initially dissolved at the micelle–water interface, experiences a gradually increasing electrostatic attraction and is therefore systematically dragged deeper inside the micellar corona, as the CTAC composition is increased in the mixed micellar systems. Consequently, the ET rate of the C343–DMAN pair undergoes a large enhancement in the studied mixed micellar systems with the increasing CTAC to pluronic molar ratio. The present strategy of modulating ET reactions using such composite supramolecular assemblies can find applications in areas where bimolecular ET is an integral reaction step.
Introduction
Electron transfer (ET) reactions in microheterogeneous media are of immense importance as they are directly involved in many practical applications, for example, in solar energy conversion, photovoltaics, biotechnology, information storage, solid state electronics, molecular electronics, sensing, catalysis, and many others.1–7 Due to such importance, ET reactions in various microheterogeneous assemblies have been investigated very extensively with the ultimate aim of achieving control over the dynamics, energetics and mechanism of the ET processes.5–24 In microheterogeneous media, the degree of hydration (i.e. micropolarity) not only determines the rate of the solvent relaxation process but also plays an important role in determining the microviscosity for the reaction environment. While the solvent relaxation rate would directly determine the contribution of solvent reorganization energy towards the free energy of activation for the ET reaction in such media, the microviscosity and the reactant entanglement with surfactant chains in such systems will in effect control the diffusion of the reactants and also the effective separation among the reacting donor–acceptor (D–A) pairs.12–14,25–28 Logically the above effects in relation to the ET reactions can be adjusted to a significant extent by applying suitable strategies that help in modulating the microenvironments of the micellar systems. Two important approaches in this regard can be: (i) addition of an electrolyte in the micellar solution and (ii) changing temperature or pressure of the solution.29–40 Addition of an electrolyte invariably increases the degree of hydration of a micelle,29–34 which in turn favours the ET reaction. The presence of high concentration of electrolyte, however, can cause a large change in the micellar shape, often leading to the undesired phase separation.31–34 Changing temperature can definitely induce significant changes in the micellar microenvironments.35 However, occurrence of a cloud point and large changes in the surfactant solubility with changing temperature often restrict the temperature range suitable for ET studies.36–38 Though micellar microenvironments can also be modified by changing the solution pressure, this methodology needs very specialized and tedious experimental arrangements.39,40Another approach, rather simple and quite effective in modulating the microenvironment of a micelle, is the use of composite micellar systems formed by the combination of pluronic copolymers and conventional surfactants.41–48 Pluronics are the tri-block copolymers, made up of poly-ethylene oxide (EO) and poly-propylene oxide (PO) blocks, with a general formula (EO)n–(PO)m–(EO)n. Pluronic copolymers have been the substances of intense research in recent times due to their unique solution behavior49–54 and extensive industrial applications.53–60 Micelle formation for pluronics beyond their critical micellar temperature (CMT) is guided by the differential solubility of poly-EO and poly-PO blocks in water.37,49–56 Thus, in these micelles, the more hydrophobic PO blocks constitute the micellar core and the more hydrophilic EO blocks constitute their hydrated corona. Since tri-block copolymers with a wide range of sizes for their poly-EO and poly-PO blocks are available,41–60 it is easy to obtain micelles with largely varying dimensions of both the core and the corona. Consequently, pluronic micelles can provide a wide range of microenvironments to dissolve solutes of largely different physicochemical characteristics and thus can find many useful applications in different areas.41–60
The combination of a pluronic copolymer and a conventional ionic surfactant can form unique mixed micellar systems displaying very distinct characteristics.41–48,61–66 In these systems, the added surfactants are incorporated into the basic pluronic micellar structure with hydrophobic chains of the surfactants embedded into the nonpolar core and the charged head groups of the surfactants residing at the peripheral region of the core, protruding into the hydrated corona region.41–43,61–66 Due to this unique structural arrangement, a charged layer is developed deep inside these mixed micelles and accordingly an ionic solute, having a charge opposite to that of the charged layer and initially localized at the micelle–water interface in the absence of the ionic surfactant, is gradually attracted by the electrostatic effect and hence is dragged deeper into the micelle, a process that can be controlled suitably by changing the surfactant to pluronic molar ratios of the mixed micellar systems.45–48,66
The aim of the present study is to explore the above feature of the ionic surfactant–copolymer mixed micellar systems for possible tuning of a bimolecular ET reaction. In this study, a cationic surfactant, namely, cetyltrimethylammonium chloride (CTAC), has been used along with two pluronic copolymers, namely, P123 (EO20–PO70–EO20) and F88 (EO103–PO39–EO103), having largely different sizes of their poly-EO and poly-PO blocks, such that a cationic charge layer is developed inside the mixed micelles. An anionic fluorophore, the deprotonated coumarin-343 (C343) dye, has been used as the electron acceptor (A) in combination with a neutral electron donor (D), N,N-dimethylaniline (DMAN), to study the ET reaction following fluorescence quenching measurements. The versatility of the coumarin–amine systems in carrying out efficient ET reactions and thus to understand many inside of the mechanisms and dynamics of these reactions has been well documented in the literature.5–24,28,30,67,68 In the present study, we expect that in these mixed micelles the location of the anionic C343 will be gradually changed on changing the surfactant to pluronic ratios and accordingly there will be a modulation in the observed ET rate.
Methods and materials
P123 (Aldrich), CTAC (Aldrich), and C343 dye (Exciton; Laser grade) were used as received. F88 was a gift from the BASF Corporation, Edison, NJ, USA. DMAN (Spectrochem, India) was purified by vacuum distillation just before use. Nanopure water having a conductivity <0.1 μS cm−1 from a Millipore Elix-3/A10 system was used for solution preparation. Stock solution of P123 (20% w/v) was prepared by dissolving a weighed amount of the copolymer in a requisite amount of water in a sealed container, keeping the mixture overnight under refrigeration. Similarly, stock solution of F88 (10% w/v) was prepared by dissolving a weighed amount of F88 in a requisite amount of water, keeping the mixture stirred for 24 h at room temperature. A stock CTAC solution was similarly prepared by dissolving the required amount of the surfactant in water. P123–CTAC and F88–CTAC composite micellar systems were finally prepared by using the stock CTAC and pluronic solutions, maintaining the pluronic concentrations as 10% w/v for P123 and 5% w/v for F88, while the CTAC concentration was gradually varied as required. C343 was directly added to the micellar solutions and the dye concentration in the experimental solution was adjusted by appropriate dilution, keeping its concentration quite low (<5 μM), to avoid multi-occupancy of the dye in a micelle. For quenching experiments, stock DMAN solution was first prepared by adding a required volume of the neat amine to a known volume of the C343 solution in the concerned micellar system. A corresponding reference solution containing only the dye with the same concentration but without any DMAN was also accordingly made. These two experimental solutions were finally mixed in suitable proportions to carry out the quenching measurements with varying DMAN concentration but keeping the dye concentration as well as the surfactant to pluronic molar ratio constant for each set of the experiments. Since P123 and F88 have largely different CMT values,50,51 measurements in the present study were carried out at 25 °C in 10% P123 solution (CMT ∼ 13 °C) and at 38 °C in 5% F88 solutions (CMT ∼ 31 °C), to ascertain complete micelle formation of the pluronics in the studied solutions. For the P123 and F88 systems, the relevant micellar parameters obtained from the literature15,16 are listed in Table 1. The molecular structures of the C343 acceptor and DMAN donor and also the molecular formulae of the CTAC surfactant and P123 and F88 pluronic copolymers, used in the present study, are shown in Scheme 1 for quick visualization.| Pluronic system (average MW) | [P]t/mM (w/v) | N agg | CMC/mM (w/v) | r m/Å | r c/Å |
|---|---|---|---|---|---|
P123 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) 400) |
17.3 (10%) | 86 | 0.052 (0.03%) | 57.7 | 52.0 |
| F88 (5750) | 4.39 (5%) | 62 | 0.246 (0.28%) | 84 | 38 |
 | ||
| Scheme 1 Molecular structures of the acceptor (C343), donor (DMAN), block co-polymers (P123 and F88) and surfactant (CTAC) used in the present study. | ||
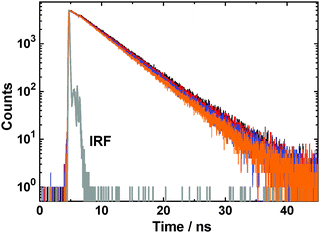
Ground state absorption and steady-state (SS) fluorescence measurements were carried out using a JASCO UV-vis spectrophotometer (model V-530, Tokyo, Japan) and a Hitachi spectrofluorometer (model F-4010, Tokyo, Japan), respectively. Fluorescence quantum yield (Φf) values for the C343 dye under different solution conditions were estimated following a comparative method27 using the dye in aqueous solution as the reference (Φf = 0.7).69 Time-resolved (TR) fluorescence measurements were carried out using a time-correlated single photon counting (TCSPC)70,71 spectrometer from Horiba Jobin Yvon IBH, UK, where the samples were excited using a 374 nm diode laser (repetition rate 1 MHz, pulse width ∼100 ps) and fluorescence decays were collected at right angle geometry using a MCP PMT detector (IBH, Scotland, UK). All the TR measurements were carried out using a fluorescence analyzer at magic angle orientation with respect to the vertically polarized excitation beam to avoid any rotational depolarization effect of the dye on the observed fluorescence decays. Measured decays were analyzed using DAS-6 re-convolution software obtained from IBH. The instrument response function (IRF) for the present TCSPC setup was recorded by replacing the sample cell with a dilute scattered solution (TiO2 suspension in water) and used in the re-convolution analysis. A typical IRF for the present setup is ∼110 ps at full-width half-maximum (FWHM) and the shortest lifetime measurable using re-convolution analysis is about 30 ps.70,71 As required in different cases, the decays were fitted either as a single-exponential or a bi-exponential function, expressed in general as,
 | (1) |
Results and discussion
1.1. Absorption and fluorescence characteristics of C343 dye
The fluorophore, C343, which is also the electron acceptor in the present study, can exist either in the neutral or in the anionic form, depending on the pH of the solution.46–48,66,72–75 To characterize the prototropic form of the C343 dye that exists in the experimental solutions, both absorption and SS fluorescence spectra of the dye were recorded in aqueous solution under different pH conditions and compared with those obtained in P123 and F88 micellar solutions as shown in Fig. 1. Absorption and fluorescence spectra of the dye in P123 and F88 micellar solutions resemble quite closely with those in aqueous solution at alkaline and neutral pH but differ largely as compared to those under acidic pH conditions. These results clearly suggest that C343 mainly exists in its anionic form both in P123 and F88 micellar solutions. The present assignment is also in agreement with the earlier reports showing that in different micellar solutions the C343 dye in fact exists in its anionic form.46–48,66,72–75Establishing that C343 exists in its anionic form both in P123 (10% w/v) and F88 (5% w/v) micelles, the SS emission spectra of the dye were recorded in the mixed micellar solutions with gradually increasing concentration of CTAC but keeping the pluronic concentration constant. The observed results in the CTAC–P123 system are shown in Fig. 2. Similar results were also obtained in the CTAC–F88 system. As indicated in Fig. 2, emission spectra of the dye gradually shift toward shorter wavelengths as the CTAC content is increased in the pluronic solutions. The observed results clearly suggest that in the mixed micelles there is a gradual decrease in the micropolarity around the probe dye as the CTAC concentration is increased in the solution. As discussed earlier, addition of CTAC to P123 or F88 pluronic solutions results in the formation of unique mixed micelles where a positively charged layer is developed deep inside the micellar phase.41–48,61–66 Accordingly, the anionic dye C343, which is expectedly localized at the micelle–water interface initially in pure pluronic micelles, experiences an electrostatic attraction due to the formation of mixed micelles in the presence of CTAC and thus gradually shifted its location deeper inside the micellar corona as the CTAC concentration is increased. Since the extent of hydration would be much lower at the deeper region of the micellar corona than that at the micelle–water interface region, the shifted location of the dye is clearly manifested by the observed blue shift in the emission spectra with the increasing CTAC concentration in the mixed micellar systems.41–43,61–66,72–75Table 2 lists the relevant absorption and fluorescence parameters of the C343 dye in aqueous solution under different pH conditions and also in pure pluronic micelles and in typical CTAC–pluronic mixed micellar systems, as investigated in the present study.
| Solution | λ maxabs/nm | ε maxabs/M−1 cm−1 | λ maxfl/nm | Φ f | τ f/ns |
|---|---|---|---|---|---|
| Aqueous, pH 6.8 | 428 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
492 | 0.70 | 4.48 |
| Aqueous, pH 9.3 | 427 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
491 | 0.71 | 4.49 |
| Aqueous, pH 3.2 | 455 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
499 | 0.65 | 4.25 |
| P123 (10% w/v) | 425 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
487 | 0.67 | 4.33 |
| CTAC–P123 (mol. ratio 0.5) | 425 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
482 | 0.66 | 4.32 |
| F88 (5% w/v) | 426 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
489 | 0.69 | 4.44 |
| CTAC–F88 (mol. ratio 0.5) | 426 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
482 | 0.68 | 4.37 |
 | ||
| Fig. 2 Normalized steady-state emission spectra of C343 in 10% w/v P123 at CTAC to P123 molar ratios of: 0.0 (black), 0.1 (blue), 0.3 (green) and 0.5 (orange). | ||
TR fluorescence studies for the C343 dye in the two mixed micellar systems were carried out as a function of the changing CTAC concentration, keeping the pluronic concentration constant. Observed fluorescence decays for the C343 dye in CTAC–P123 mixed micelles at different CTAC to pluronic molar ratios are shown in Fig. 3. Similar results were obtained in CTAC–F88 mixed micelles. Observed decays in these cases were seen to fit well with a single exponential function and the fluorescence lifetime (τf) values thus obtained are listed in Table 2. As indicated from these results, there is no appreciable change in the fluorescence decays of the dye with the changing CTAC concentration in either of the CTAC–pluronic mixed micelles.
 | ||
| Fig. 3 Fluorescence decays of the C343 dye in 10% w/v P123 at different CTAC to P123 molar ratios: 0.0 (black), 0.1 (blue), 0.3 (green) and 0.5 (orange). | ||
The TR results observed in the studied mixed micelles are apparently not in accordance with the SS fluorescence results where a reasonable blue shift was observed in the emission spectra indicating a gradual reduction in the micropolarity around the dye in the mixed micelles with an increase in the CTAC concentration (cf.Fig. 2). The observed TR results can, however, be expected if the fluorescence decays of the dye are inherently less sensitive to the polarity of the solvents such that the extent of the micropolarity change resulted from the changing CTAC concentration is not sufficient to cause any observable change in the observed decays. To explore this aspect further, we carried out ground state absorption, SS fluorescence and TR fluorescence measurements for the C343 dye in different water–ethanol (EtOH) solvent mixtures, with varying co-solvent compositions. In all these cases, the solution was made reasonably alkaline to ascertain the existence of the dye in its anionic form. It is found that while the fluorescence decays show only a nominal change with the changing solvent composition (cf.Fig. 4), the absorption and fluorescence spectra show quite significant changes, displaying a gradual blue shift in the spectra as the ethanol composition is increased in the solvent mixture (cf.Fig. 5). From these results it is evident that the fluorescence decays of the C343 dye are indeed not that sensitive to the solvent polarity, though the absorption and fluorescence spectra of the dye show quite significant changes on changing the solvent polarity.
1.2. Absorption and fluorescence studies on the interaction of C343 dye with DMAN quencher
Ground state absorption measurements indicate that the absorption spectra of the C343 dye in pure P123 and F88 micelles as well as in CTAC–pluronic mixed micellar systems remain very similar both in the absence and in the presence of the DMAN quencher. Moreover, the spectral feature in all these cases corresponds to that of the anionic form of the dye.46–48,66,72–75 Typical results in P123 micellar solution are shown in inset A of Fig. 6. The observed results suggest that there is no ground-state complex formation between C343 and DMAN in any of these microheterogeneous systems studied.10–20,27In the SS fluorescence measurements, even though the spectral characteristics of the dye do not show any observable change in the presence of the DMAN quencher, the fluorescence intensity gradually decreases on increasing the DMAN concentration (cf.Fig. 6). The observed results indicate that there is a strong quenching for the excited C343 dye by the DMAN quencher and the quenching in the present systems does not involve any excited state complex or exciplex formation.10–20,27 To be mentioned that in the literature there are extensive reports, suggesting and demonstrating experimentally the involvement of photoinduced ET (PET) from ground state amine donors to excited coumarin acceptors as the mechanism for the strong fluorescence quenching observed in the coumarin–amine systems.10–24,67,68,76–79 Drawing an analogy, thus, we attribute the observed fluorescence quenching in the present C343–DMAN system is due to the PET process.
To estimate the fluorescence quenching rates for the present C343–DMAN system, we tried to correlate the extent of SS fluorescence quenching with the quencher (Q) concentration used following the widely employed Stern–Volmer relation.26,27,70,71
 | (2) |
In micellar media, the reactant molecules are preferentially solubilized within a very small region of the microhetrogeneous systems.10–24 Therefore, even under the conditions where the bulk estimate for the quencher concentration apparently seems quite low, the actual localized concentration of the quencher can be significantly high such that a good fraction of the fluorophore molecules will already be in close proximity with the quenchers at the moment of their excitation.10–24 As these close proximity fluorophore–quencher pairs can lead to an unusually fast quenching interaction without involving any diffusion of the reactants, the observed SS fluorescence quenching will be much stronger than otherwise expected under normal diffusional quenching kinetics conditions.26,27 Accordingly, the I0/I vs. [Q] plots should show a positive deviation as observed in the present study.26,27 Obviously, such positive deviations in the SS fluorescence quenching results, as invariably observed for bimolecular ET reactions in all kinds of microheterogeneous media, arise because of the fact that the reactants are confined within a small region of the supramolecular systems.10–24,80–84 Since with such deviations in the I0/I vs. [Q] plots the KSV values cannot be estimated with sufficient accuracy (even on using the initial slopes for the plots), we did not use the observed SS fluorescence quenching results for any quantitative estimation of the quenching kinetics. In the present study, thus, we entirely relied on the TR fluorescence results to estimate the quenching kinetics, as are discussed in the following section.
1.3. Time-resolved fluorescence studies on the interaction of C343 with DMAN
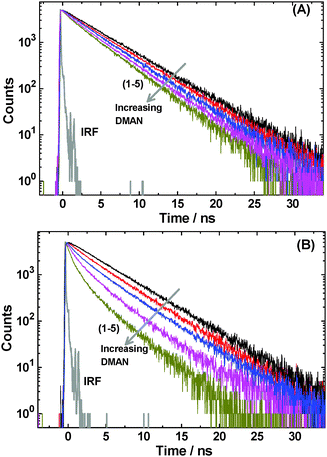
TR fluorescence studies on the interaction of the C343 dye with the DMAN quencher were carried out in pure pluronic micelles as well as in CTAC–pluronic mixed micellar systems, following quencher concentration dependent changes in the fluorescence decays of the dye. It is observed that the decays gradually become faster as the DMAN concentration is increased in the solution. These results are in accordance with the SS fluorescence quenching results and suggest an efficient ET interaction for the excited C343 dye with the ground state DMAN quencher. Representative decays observed on changing the DMAN concentration in pure P123 micelle and in a selected CTAC–P123 mixed micelle are shown in Fig. 7A and B, respectively. Similar results in pure F88 micelle and in a typical CTAC–F88 mixed micellar system are shown in Fig. 8A and B, respectively.As mentioned in Section 1.1, in the absence of the quencher, the fluorescence decays of the dye in both pure pluronic and CTAC–pluronic micelles are single exponential in nature. In the presence of the DMAN quencher, however, the decays of the dye in all the micellar systems studied are found to become non-single exponential in nature. Nevertheless, these decays in the presence of the quencher could be fitted reasonably well with a bi-exponential function, at least for the concentration range of the quencher used in the present study.
An important observation from Fig. 7 and 8 is that the TR fluorescence quenching is always more efficient in the mixed micellar systems than in the corresponding pure pluronic micelles, for a given DMAN concentration. This becomes further evident as we compare the decays as a function of the increasing CTAC to pluronic molar ratios for a given DMAN concentration, as are shown in Fig. 9A and B, respectively, for CTAC–P123 and CTAC–F88 mixed micellar systems. It is also evident from Fig. 9 that the effect of the added CTAC in modulating the quenching rate is much more dominant in CTAC–F88 mixed micellar systems than in CTAC–P123 mixed micellar systems, which corroborates well with the larger poly-EO block size in F88 copolymers than that in P123.15,16,46–50,66
Fluorescence decays measured in the two mixed micellar systems at different DMAN concentrations were systematically analyzed following a bi-exponential function. To understand the extent of TR quenching for the dye with the changing DMAN concentration, the average lifetime (〈τ〉) values were estimated in each case using the following relation,10–24,70,71,80,81
 | (3) |
As indicated in Fig. 10, in both the mixed micellar systems, the 〈τ〉 value of the dye at a given DMAN concentration gradually becomes shorter as the CTAC to pluronic molar ratio is increased. These results clearly suggest that in the studied mixed micelles there is a large modulation in the ET rate for the C343–DMAN pair, achieved simply through a change in the CTAC to pluronic molar ratio. We attribute this effect to the gradual drifting of the anionic C343 dye from its initial solubilization site at the micelle–water interface to deeper into the micellar corona, due to the development of the positively charged layer inside the CTAC–pluronic mixed micelles,41–48,61–66 which can be pictorially represented by Scheme 2 for a simple visualization.
1.4. Modulations of the ET rates for the C343–DMAN system in the CTAC–pluronic mixed micellar media
With the observations presented and discussed so far, it is evident that the quenching/ET interaction between the C343 acceptor and the DMAN donor is modulated very largely by changing the CTAC to pluronic molar ratio in the studied mixed micellar systems. To have a quantitative estimate of this effect, the TR fluorescence quenching results were systematically correlated with the changing DMAN concentrations at different CTAC to pluronic molar ratios in the two mixed micellar systems studied. Since the quenching interaction in the close contact donor–acceptor pairs occurs with an unusually fast rate,13,14,24,68 much faster than the time resolution of the present TCSPC setup (∼30 ps), unlike in the SS fluorescence quenching, the reductions in the 〈τ〉 values observed in the present TR studies will not have any significant contribution from the ultrafast (or static) quenching components.10–24,68 With this assumption, the observed changes in the 〈τ〉 values as a function of DMAN concentrations in each of the studied micellar media (pure pluronic or CTAC–pluronic micelles) were correlated following the widely employed Stern–Volmer relation for TR quenching studies,26,27,70,71 as given by the following equation. | (4) |
 | ||
| Fig. 11 Stern–Volmer plots at different CTAC to pluronic molar ratios. [CTAC]/[pluronic] = 0 (blue) and [CTAC]/[pluronic] = 0.5 (red). Panel (A) P123 system and panel (B) F88 system. | ||
Fig. 12 shows the changes in the KSV values in the CTAC–pluronic mixed micellar systems as a function of the increasing CTAC to pluronic molar ratios. As indicated from this figure, in both CTAC–P123 and CTAC–F88 mixed micelles, there is a large asymptotic increase in the KSV values (about 2.6 fold in the case of CTAC–P123 and about 3.6 fold in the case of CTAC–F88 systems) with an increase in the CTAC composition and the effect apparently tends towards a saturation limit at CTAC to pluronic molar ratios of about 0.5 and 0.8, in the two respective mixed micelles. Such a large increase in the KSV values is a very intriguing and important observation and it explicitly suggests that there is a large increase in the overall ET/quenching interaction achieved just by increasing the composition of the cationic CTAC surfactant in the studied surfactant–pluronic mixed micellar systems.
 | ||
| Fig. 12 Changes in the Stern–Volmer quenching constant (KSV) as a function of CTAC to pluronic molar ratios in the CTAC–P123 (blue) and CTAC–F88 (red) supramolecular systems. | ||
One of the possible reasons for the observed increase in the KSV values for the present ET systems in the studied mixed micelles can be the likely increase in the hydration at the micellar corona due to the incorporation of the more hydrophilic CTAC surfactants into the pluronic micelles.29–38,41–48,61–66 One would expect this because the rate of an ET reaction normally increases with an increase in the solvent polarity.1–16,22–26 In one of our earlier studies on the ET reactions in surfactant–pluronic mixed micellar systems, albeit on employing the electrically neutral molecules as both the electron acceptor and the donor, it was observed that in the mixed micelles consisting of the neutral surfactant TX100 and the pluronic P123, the ET rate undergoes only a marginal change on changing the surfactant to pluronic composition.10 It was thus inferred that the hydration of the mixed micelle does not change much when TX100 is incorporated into the P123 micelle, as both TX100 and poly-EO block of P123 are of comparable hydrophobicity. On the other hand, on using ionic surfactants (e.g. anionic surfactant sodium dodecyl sulfate (SDS) or cationic surfactant CTAC) in combination with P123, it was observed that there is a significant increase in the ET rates, which was logically attributed to the increased hydration of the mixed micelles, as the ionic surfactants are more hydrophilic than the poly-EO block of the pluronic polymers.10 In that study, as both the donor and the acceptor molecules are electrically neutral, assignment of the enhanced ET rates to the increased micellar hydration was quite justified because neutral reactants will not suffer any change in their locations in the microheterogeneous systems due to the formation of the charged layers inside the mixed micelles, as the ionic surfactants are incorporated into the pluronic micelles. In the present study, however, assignment of the enhanced quenching rates to the increased micellar hydration seems to be contradicting with the observed blue shifts in the SS fluorescence spectra (cf.Fig. 2) that suggested the dye to experience a gradually reduced micropolarity with the increasing composition of CTAC in the CTAC–pluronic mixed micellar systems. It is thus expected that in the present systems the enhancement in the ET rates for the C343–DMAN pair must be due to some other effect than the normally expected reason of the increased micellar hydration.
In the present study, as the electron acceptor dye C343 is in the anionic form, in the absence of CTAC, the dye is expected to be preferentially localized at the micelle–water interface region of the pure pluronic micelles. With the inclusion of CTAC into these micelles, as a positively charged layer is developed inside the mixed micelles,41–48,61–66 the anionic C343 dye is electrostatically attracted by this positively charged layer and consequently the dye is gradually dragged deeper inside the micellar corona (cf.Scheme 2), causing it to encounter a relatively lower micropolarity with increasing CTAC composition in the mixed micelle (cf.Fig. 2). With this situation, the enhanced ET/quenching rates for the present donor–acceptor pair must not be related to the increased micellar hydration as suggested in the earlier study,10 but is certainly associated with the changing location of the dye in the present mixed micellar systems,45–48,66 as the CTAC composition is gradually increased.
Considering that the dye gradually moves deeper inside the micellar corona, it is quite expected that its effect on the observed ET/quenching rates will eventually get saturated at a high CTAC concentration, when the dye is already close to the positively charged layer. This is in fact very clearly indicated from the trends of the plots shown in Fig. 12. Moreover, based on this consideration and taking into account that the poly-EO block size of pluronic F88 is much larger than that of P123, it is expected that the saturation of the quenching rates would eventually reach at a relatively higher CTAC composition for the CTAC–F88 mixed micelle than the CTAC–P123 mixed micelle, as indicated from Fig. 10 and 12, because the corona thickness is much larger for the former case than the latter (cf.Table 1). These results are thus in direct support of our interpretation that the enhancement in the overall ET/quenching rates in the present mixed micellar systems is mainly due to the changing location of the dye with the changing CTAC composition.
Since the micropolarity around the C343 dye is indicated to decrease with an increase in the CTAC to pluronic molar ratio in the mixed micelles, the enhancement in the ET/quenching rates for the C343–DMAN pair was apparently unexpected under the normal circumstances. We feel that a major contributor to this increase in the quenching rate arises due to the inhomogeneous distribution of the DMAN quencher in the micellar corona region. Being neutral and relatively more hydrophobic in nature, it is expected that DMAN quenchers will be more solubilized at the inner region of the micellar corona than at the micelle–water interface. Therefore, as the dye is gradually dragged inside the micellar corona, it consequently encounters a successively higher quencher concentration and hence undergoes a more efficient quenching interaction, which is clearly indicated by the plots in Fig. 10 and 12. Though such a situation of the inhomogeneous solubility seems to be very logical for a neutral and relatively less polar reactant (DMAN in the present case) in a micellar media, to the best of our knowledge no experimental evidence for such a phenomenon has so far been reported in the literature. The results in the present study thus exemplify this new insight of the fluorescence quenching process in the micellar and other microheterogeneous media where inhomogeneous solubility of the reactants can significantly influence the observed quenching rates. For the studied ET system, one more important point to be mentioned here is that there could be an additional contribution towards the observed enhancement in the ET rates with the increasing CTAC to pluronic ratios arising due to the changing redox characteristic of the dye with its changing location in the mixed micellar systems, as reported earlier.48 In the present study, however, it was not possible to distinguish and quantify the contributions of the above two effects on the observed enhancements in the ET rates with the changing CTAC to pluronic molar ratios.
As indicated from Fig. 12, for a particular CTAC to pluronic molar ratio, the KSV value is much higher in the CTAC–F88 system than in the CTAC–P123 system. The higher reaction rate certainly indicates that the microenvironment in the corona region of CTAC–F88 mixed micelle is more conducive for the ET interaction than that of the CTAC–P123 mixed micelle. We attribute this to the greater hydration of F88 micellar corona than that of P123. For F88, the two terminal poly-EO blocks contain 103 EO units each in comparison to only 20 EO units present in each of the two terminal poly-EO blocks in P123. Therefore, due to larger number of EO units, the corona regions of the pure and mixed micelles of F88 pluronic are much larger in thickness and hence much more hydrated compared to those of the micelle and mixed micelles of pluronic P123.29–38,41–48 Accordingly, the ET reaction in the C343–DMAN pair occurs with much higher propensity in CTAC–F88 mixed micellar systems than in the CTAC–P123 mixed micellar systems.1–16,22–26,61–66
It is reported in the literature that for ionic surfactant–pluronic supramolecular assemblies both the hydrodynamic and core radii of the micelles decrease to some extent with an increase in the surfactant to pluronic molar ratio.10,16,41,47,62,64 In fact, in some of our earlier studies this aspect has been investigated for CTAC–P123 and CTAB–F88 (CTAB: cetyltrimethylammonium bromide) mixed micellar systems using small angle neutron scanning (SANS) measurements and the reduction in the micellar size is found to be about 10–15% for the surfactant to pluronic molar ratio of about 0.5.10,47 For the present CTAC–pluronic mixed micellar systems also we expect quite a similar kind of changes in the micellar sizes with an increase in the CTAC to pluronic molar ratios, though we did not go for any explicit measurements for the micellar sizes in the present study. Moreover, as mentioned earlier, the donor DMAN is supposed to have a non-uniform distribution inside the micellar corona, being solubilized more at the deeper region of the corona than at the micelle–water interface. Thus, for the present systems, at any given bulk DMAN concentration ([DMAN]t), the C343 dye encounters a gradually changing effective DMAN concentration ([DMAN]eff) in the course of its changing location in the micellar corona with the increasing CTAC to pluronic ratio. With this complex situation, the actual [DMAN]eff is difficult to be estimated at each stage of the experimental conditions and hence the Stern–Volmer analysis (cf.eqn (4)) of the TR fluorescence quenching data using [DMAN]eff is extremely difficult. Therefore, for the present systems, the Stern–Volmer analysis of the TR fluorescence quenching data were effectively carried out in terms of [DMAN]t as directly used in the experimental solutions. Consequently, the KSV values estimated from these analyses are the apparent Stern–Volmer constants, which would be related to the true KSV values (KtrueSV) by the following relation,
 | (5) |
 | (6) |
Following eqn (5) and (6) the kq values in pure P123 and F88 micelles are estimated to be about 2.0 × 107 M−1 s−1 and 1.9 × 108 M−1 s−1, respectively, which are distinctly lower than the expected bimolecular diffusional rate constants (kd) in P123 (kd ∼ 1.5 × 108 M−1 s−1) and F88 (kd ∼ 2.4 × 108 M−1 s−1) micelles as reported earlier.15,16 Interestingly, the kq values estimated for the present ET system are about one order of magnitude lower in comparison to the typical kq values observed for the ET systems involving different neutral coumarin dyes in combination with the DMAN quencher in the same P123 and F88 micellar solutions.15,16 The present results thus clearly indicate that in pure pluronic micelles the ET reaction involving the anionic C343 dye is quite less efficient in comparison to that involving neutral coumarin dyes. This we attribute to the preferential solubilization of the anionic C343 dye at the micelle–water interface where population of the neutral DMAN quencher is quite low and hence a less proficient ET reaction occurs in this case as compared to that of the neutral coumarin dyes that preferentially reside well within the micellar corona.
In the present mixed micellar assemblies, the kq values estimated following eqn (5) and (6) also clearly indicate an asymptotic increase, a trend very similar to that shown in Fig. 12, as the CTAC to pluronic molar ratio is gradually increased. The saturation range for the kq values is thus estimated to be about 5.1 × 107 M−1 s−1 in CTAC–P123 and about 7.2 × 108 M−1 s−1 in CTAC–F88 mixed micellar systems. Interestingly, these values are quite close to the typical kq values observed earlier in pure P123 and F88 micellar solutions involving different neutral coumarin dyes in combination with the DMAN quencher,15,16 providing a support to our proposition that the anionic C343 dye is being dragged deeper inside the micellar corona as the CTAC composition is increased in the mixed micellar systems.
As already indicated, both the KSV and kq values estimated in the present study are actually the apparent values because the true [DMAN]eff values could not be estimated in the present study. In spite of this limitation, the observed KSV and kq values are still informative enough, displaying an intriguing and exceptionally large increase in the quenching rates for the studied ET reaction in the CTAC–pluronic mixed micellar media just by increasing the CTAC to pluronic molar ratios. The present results clearly demonstrate that the kinetics of the ET reactions can be modulated very significantly with appropriate selection of the electron donor–acceptor systems and the suitable combination of the ionic surfactant and pluronic polymer in the mixed micellar media, simply by changing the composition of the composite microheterogeneous system. Such modulations in the ET rates can have profound implications in the applied areas where bimolecular ET reactions are involved as the elementary steps in the concerned reaction mechanisms.
Conclusions
The present study demonstrates a large modulation in the rates of the ET reaction involving an anionic electron acceptor (C343) and a neutral electron donor (DMAN), achieved simply by changing the composition of a cationic surfactant (CTAC) to a pluronic polymer (P123 or F88) in the surfactant–pluronic mixed micellar assemblies. It is understood that the location of the anionic acceptor C343 in the studied mixed micelles is gradually changed as the composition of cationic surfactant CTAC is increased in the mixed micellar systems. The observed results are interpreted on the basis of the reported structural motifs of the mixed micelles where ionic surfactants are embedded with their hydrocarbon chains grafted into the micellar core leaving their ionic head groups projected out of the core protruding into the micellar corona. Accordingly, a positively charged layer is developed deep inside the mixed micelle which attracts the anionic C343, gradually dragging the dye deeper inside the micellar corona, as the CTAC composition is increased in the system. As DMAN donor is a neutral and relatively hydrophobic molecule, it is solubilized more preferentially at the deeper region of the micellar corona than at the micelle–water interface. Accordingly, while the C343 acceptor is dragged into the micellar corona, it gradually encounters an increased concentration of DMAN and hence undergoes an enhanced fluorescence quenching mediated by ET interaction. The observed results in the present study are very interesting and exciting because they demonstrate a large modulation in the ET rates simply by changing the surfactant to pluronic molar ratios in the mixed micellar systems, a strategy very convenient to implement for a desired outcome from an ET reaction. We strongly feel that the present approach of modulating the ET rates will find uses in different applied areas where bimolecular ET reactions are involved as the fundamental reaction steps.Acknowledgements
The authors are thankful to Dr B. S. Tomar, Head, RACD, Dr D. K. Palit, Head, RPCD, Dr K. L. Ramakumar, Director, Radiochemistry & Isotope Group, and Dr B. N. Jagatap, Director, Chemistry Group, for their constant encouragement and support. The authors are grateful to Dr S. Nath of RPCD, BARC, for his kind help in providing the F88 sample that he had obtained from BASF Corporation, Edison, USA, as a gift and for his kind assistance in some of the initial measurements in the present study.Notes and references
- Electron transfer from isolated molecules to biomolecules. Advances in Chemical Physics, ed. J. Jortner and M. Bixon, Wiley, New York, 1999, vol. 106 & 107 Search PubMed.
- H. Petek and J. Zhao, Chem. Rev., 2010, 110, 7082–7099 CrossRef CAS PubMed.
- D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2001, 34, 40–48 CrossRef CAS PubMed.
- M. Hambourger, G. F. Moore, D. M. Kramer, D. Gust, A. L. Moore and T. A. Moore, Chem. Soc. Rev., 2009, 38, 25–35 RSC.
- M. Gratzel, Heterogeneous photochemical electron transfer, CRC Press, Boca Raton, FL, 1989 Search PubMed.
- K. D. Jordan and M. N. Paddon-Row, Chem. Rev., 1992, 92, 395–410 CrossRef CAS.
- D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2009, 42, 1890–1898 CrossRef CAS PubMed.
- S. F. Swallen, K. Weidemaier, H. L. Tavernier and M. D. Fayer, J. Phys. Chem., 1996, 100, 8106–8117 CrossRef CAS.
- S. Fukuzumi, M. Nishimine, K. Ohkubo, N. V. Tkachenko and H. Lemmetyinen, J. Phys. Chem. B, 2003, 107, 12511–12518 CrossRef CAS.
- M. Kumbhakar, S. Dey, P. K. Singh, S. Nath, A. K. Satpati, R. Gangully, V. K. Aswal and H. Pal, J. Phys. Chem. B, 2011, 115, 1638–1651 CrossRef CAS PubMed.
- M. Kumbhakar, S. Nath, T. Mukherjee and H. Pal, J. Chem. Phys., 2005, 123, 034705 CrossRef PubMed.
- M. Kumbhakar, S. Nath, T. Mukherjee and H. Pal, J. Chem. Phys., 2004, 120, 2824–2834 CrossRef CAS PubMed.
- M. Kumbhakar, P. K. Singh, S. Nath, A. C. Bhasikuttan and H. Pal, J. Phys. Chem. B, 2008, 112, 6646–6652 CrossRef CAS PubMed.
- M. Kumbhakar, P. K. Singh, S. Nath, A. K. Satpati and H. Pal, J. Phys. Chem. B, 2010, 114, 10057–10065 CrossRef CAS PubMed.
- A. K. Satpati, M. Kumbhakar, S. Nath and H. Pal, J. Phys. Chem. B, 2007, 111, 7550–7560 CrossRef CAS PubMed.
- P. Verma, S. Nath, P. K. Singh, M. Kumbhakar and H. Pal, J. Phys. Chem. B, 2008, 112, 6363–6372 CrossRef CAS PubMed.
- T. K. Mukherjee, P. P. Mishra and A. Datta, Chem. Phys. Lett., 2005, 407, 119–123 CrossRef CAS PubMed.
- S. Ghosh, K. Sahu, S. K. Mondal, P. Sen and K. Bhattacharyya, J. Chem. Phys., 2006, 125, 054509 CrossRef PubMed.
- C. Tablet, I. Matei and M. Hillebrand, J. Mol. Liq., 2011, 160, 57–62 CrossRef CAS PubMed.
- A. Chakraborty, D. Seth, P. Setua and N. Sarkar, J. Chem. Phys., 2008, 128, 204510 CrossRef PubMed.
- L. Chen, P. D. Wood, A. Mnyusiwalla, J. Marlinga and L. J. Johnston, J. Phys. Chem. B, 2001, 105, 10927–10935 CrossRef CAS.
- K. Glusac, A. Goun and M. D. Fayer, J. Chem. Phys., 2006, 125, 054712 CrossRef PubMed.
- M. Lopez-Lopez, F. Sanchez and M. Marchena, Prog. React. Kinet. Mech., 2012, 37, 203–248 CrossRef CAS.
- M. Kumbhakar and H. Pal, ScienceJet, 2015, 4:149, 01–17 Search PubMed.
- A. Paul and A. Samanta, J. Phys. Chem. B, 2007, 111, 1957–1962 CrossRef CAS PubMed.
- G. J. Kavarnos, Fundamentals of photoinduced electron transfer, VCH Publishers, New York, 1993 Search PubMed.
- J. R. Lakowicz, Principle of fluorescence spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- K. Yoshihara, K. Tominaga and Y. Nagasawa, Bull. Chem. Soc. Jpn., 1995, 68, 696–712 CrossRef CAS.
- I. D. Charlton and A. P. Doherty, J. Phys. Chem. B, 2000, 104, 8327–8332 CrossRef CAS.
- M. Kumbhakar and R. Ganguly, J. Phys. Chem. B, 2007, 111, 3935–3942 CrossRef CAS PubMed.
- R. Ganguly and V. K. Aswal, J. Phys. Chem. B, 2008, 112, 7726–7731 CrossRef CAS PubMed.
- T. Goel, M. Kumbhakar, T. Mukherjee and H. Pal, J. Photochem. Photobiol., A, 2010, 209, 41–48 CrossRef CAS PubMed.
- D. Bendedouch and S.-H. Chen, J. Phys. Chem., 1984, 88, 648–652 CrossRef CAS.
- K. Kalyanasundaram, M. Gratzel and J. K. Thomas, J. Am. Chem. Soc., 1975, 97, 3915–3922 CrossRef CAS.
- M. Kumbhakar, T. Goel, T. Mukherjee and H. Pal, J. Phys. Chem. B, 2004, 108, 19246–19254 CrossRef CAS.
- K. Kalyansundaram, Photochemistry in microheterogeneous systems, Academic Press, Orlando, FL, 1987 Search PubMed.
- B. Chu and Z. Zhou, in Surfactant science series, ed. V. M. Nace, Marcel Dekker, New York, 1996, vol. 60, p. 67 Search PubMed.
- R. Ganguly, M. Kumbhakar and V. K. Aswal, J. Phys. Chem. B, 2009, 113, 9441–9446 CrossRef CAS PubMed.
- K. Hara, N. Baden and O. Kajimoto, J. Phys.: Condens. Matter, 2004, 16, S1207–S1214 CrossRef CAS.
- T. Molotsky, N. Koifman and D. Huppert, J. Phys. Chem. A, 2002, 106, 12185–12190 CrossRef CAS.
- J. Jansson, K. Schillen, G. Olofsson, R. C. daSilva and W. Loh, J. Phys. Chem. B, 2004, 108, 82–92 CrossRef CAS.
- J. Jansson, K. Schillen, M. Nilsson, O. Soderman, G. Fritz, A. Bergmann and O. Glatter, J. Phys. Chem. B, 2005, 109, 7073–7083 CrossRef CAS PubMed.
- R. Ganguly, V. K. Aswal and P. A. Hassan, J. Phys. Chem. B, 2006, 110, 9843–9849 CrossRef CAS PubMed.
- D. Chen, Z. Li, C. Yu, Y. Shi, Z. Zhang and B. Tu, Chem. Mater., 2005, 17, 3228–3234 CrossRef CAS.
- K. S. Mali, G. B. Dutt and T. Mukherjee, J. Phys. Chem. B, 2007, 111, 5878–5884 CrossRef CAS PubMed.
- P. K. Singh, M. Kumbhakar, H. Pal and S. Nath, J. Phys. Chem. B, 2009, 113, 1353–1359 CrossRef CAS PubMed.
- P. K. Singh, M. Kumbhakar, R. Ganguly, V. K. Aswal, H. Pal and S. Nath, J. Phys. Chem. B, 2010, 114, 3818–3826 CrossRef CAS PubMed.
- P. K. Singh, A. K. Satpati, M. Kumbhakar, H. Pal and S. Nath, J. Phys. Chem. B, 2008, 112, 11447–11450 CrossRef CAS PubMed.
- E. D. Goddard, J. Colloid Interface Sci., 2002, 256, 228–235 CrossRef CAS.
- P. Alexandridis, J. F. Holzwarth and T. A. Hatton, Macromolecules, 1994, 27, 2414–2425 CrossRef CAS.
- G. Wanka, H. Hoffmann and W. Ulbricht, Macromolecules, 1994, 27, 4145–4159 CrossRef CAS.
- K. Nakashima and P. Bahadur, Adv. Colloid Interface Sci., 2006, 123–126, 75–96 CrossRef CAS PubMed.
- M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2013, 15, 10580–10611 RSC.
- M. Ramanathan, Y.-C. Tseng, K. Ariga and S. B. Darling, J. Mater. Chem. C, 2013, 1, 2080–2091 RSC.
- P. Bahadur and G. Riess, Tenside, Surfactants, Deterg., 1991, 28, 173–179 CAS.
- I. R. Schmolka, J. Am. Oil Chem. Soc., 1982, 59, 322–327 CrossRef CAS.
- A. Kabanov, J. Zhu and V. Alakhov, Adv. Genet., 2005, 53, 231–261 CAS.
- K. Nakashima and P. Bahadur, J. Controlled Release, 2002, 82, 189–212 CrossRef.
- M. Yokoyama, Crit. Rev. Ther. Drug Carrier Syst., 1992, 9, 213–248 CAS.
- K. Kataoka, A. Harada and Y. Nagasaki, Adv. Drug Delivery Rev., 2001, 47, 113–131 CrossRef CAS.
- R. C. daSilva, G. Olofsson, K. Schille’n and W. Loh, J. Phys. Chem. B, 2002, 106, 1239–1246 CrossRef.
- Y. Li, R. Xu, D. M. Bloor and J. F. Holzwarth, Langmuir, 2000, 16, 10515–10520 CrossRef CAS.
- Y. Li, R. Xu, S. Couderc, D. M. Bloor, J. F. Holzwarth and E. Wyn-Jones, Langmuir, 2001, 17, 5742–5747 CrossRef CAS.
- T. Thurn, S. Couderc, J. Sidhu, D. M. Bloor, J. Penfold, J. F. Holzwarth and E. Wyn-Jones, Langmuir, 2002, 18, 9267–9275 CrossRef CAS.
- D. Lof, M. Tomsic, O. Glatter, G. Fritz-Popovski and K. Schillen, J. Phys. Chem. B, 2009, 113, 5478–5486 CrossRef PubMed.
- P. K. Singh, M. Kumbhakar, H. Pal and S. Nath, J. Phys. Chem. B, 2008, 112, 7771–7777 CrossRef CAS PubMed.
- M. Murakami, K. Ohkubo, T. Nanjo, K. Souma, N. Suzuki and S. Fukuzumi, ChemPhysChem, 2010, 11, 2594–2605 CrossRef CAS PubMed.
- M. Kumbhakar, A. Manna, M. Sayed, A. Kumar and H. Pal, J. Phys. Chem. B, 2014, 118, 10704–10715 CrossRef CAS PubMed.
- H. N. Ghosh, J. Phys. Chem. B, 1999, 103, 10382–10387 CrossRef CAS.
- D. V. O’Connor and D. Philips, Time correlated single photon counting, Academic Press, New York, 1984 Search PubMed.
- W. Becker, Advanced time correlated single photon counting technique, Springer, New York, 2005 Search PubMed.
- R. E. Riter, E. P. Undiks and N. E. Levinger, J. Am. Chem. Soc., 1998, 120, 6062–6067 CrossRef CAS.
- D. M. Willard, R. E. Riter and N. E. Levinger, Dynamics of Polar Solvation in Lecithin/Water/Cyclohexane Reverse Micelles, J. Am. Chem. Soc., 1998, 120, 4151–4160 CrossRef CAS.
- C. D. Grant, M. R. DeRitter, K. E. Steege, T. A. Fadeeva and E. W. Castner, Langmuir, 2005, 21, 1745–1752 CrossRef CAS PubMed.
- K. Tominaga and G. C. Walker, J. Photochem. Photobiol., A, 1995, 87, 127–133 CrossRef CAS.
- S. Nad and H. Pal, J. Phys. Chem. A, 2000, 104, 673–680 CrossRef CAS.
- S. Nad and H. Pal, J. Photochem. Photobiol., A, 2000, 134, 9–15 CrossRef CAS.
- S. Nad and H. Pal, J. Chem. Phys., 2002, 116, 1658–1670 CrossRef CAS PubMed.
- S. Nad and H. Pal, J. Phys. Chem. A, 2002, 106, 6823–6831 CrossRef CAS.
- C. Ghatak, V. G. Rao, S. Mandal and N. Sarkar, Phys. Chem. Chem. Phys., 2012, 14, 8925–8935 RSC.
- A. Chakraborty, D. Seth, P. Setua and N. Sarkar, J. Chem. Phys., 2008, 128, 204510 CrossRef PubMed.
- M. Kumbhakar, S. Nath, T. Mukherjee and H. Pal, J. Indian Chem. Soc., 2010, 87, 173–187 CAS.
- S. Ghosh, D. Banik, A. Roy, N. Kundu, J. Kuchlyan and N. Sarkar, Phys. Chem. Chem. Phys., 2014, 16, 25024–25038 RSC.
- S. Sarkar, S. Mandal, R. Pramanik, C. Ghatak, V. G. Rao and N. Sarkar, J. Phys. Chem. B, 2011, 115, 6100–6110 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2015 |