Two-dimensional structure Au nanosheets are super active for the catalytic reduction of 4-nitrophenol†
Abstract
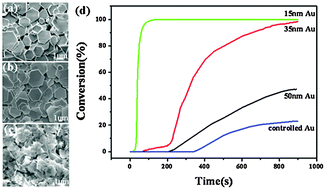
Two-dimensional structure Au nanosheets with a polygon morphology and controlled thicknesses of ∼15 nm, ∼35 nm, and ∼50 nm were successfully synthesized by a one-step solution reduction method. Scanning and transmission electron microscopy (SEM and TEM), selected area electron diffraction (SEAD) analyses, and X-ray diffraction (XRD) were used to thoroughly study the structure and the formation mechanism of the nanosheets. The catalytic activity of the Au nanosheets was investigated for the reduction of 4-nitrophenol (4-NP) by UV-visible absorption spectroscopy. Against all expectation, the Au nanosheets with such a big lateral (more than 1 μm) size exhibited superior catalytic activity on the selective reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in the presence of NaBH4. On the other hand, the catalytic activity does closely depend on the thickness of the nanosheets; that is, it decreases with increasing thickness. The reaction can be completed in less than 1 min when catalyzed by Au nanosheets about 15 nm thick. The 100% conversion efficiency was further demonstrated after two catalytic cycles with the thinnest Au nanosheets.

 Please wait while we load your content...
Please wait while we load your content...