Physical properties of high Li-ion content N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide based ionic liquid electrolytes†
Abstract
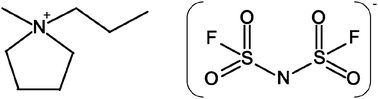
Electrolytes based on bis(fluorosulfonyl)imide (FSI) with a range of LiFSI salt concentrations were characterized using physical property measurements, as well as NMR, FT-IR and Raman spectroscopy. Different from the behavior at lower concentrations, the FSI electrolyte containing 1 : 1 salt to IL mole ratio showed less deviation from the KCl line in the Walden plot, suggesting greater ionic dissociation. Diffusion measurements show higher mobility of lithium ions compared to the other ions, which suggests that the partial conductivity of Li+ is higher at this higher composition. Changes in the FT-IR and Raman peaks indicate that the cis-FSI conformation is preferred with increasing Li salt concentration.

 Please wait while we load your content...
Please wait while we load your content...