Electronic structure and electrode properties of tetracyanoquinodimethane (TCNQ): a surface science investigation of lithium intercalation into TCNQ
Abstract
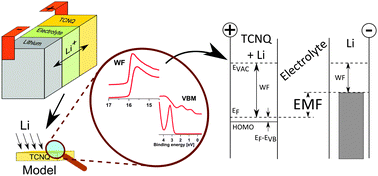
Organic materials are of interest as ion battery cathode materials because they offer advantages over inorganic cathodes such as abundant resources and a low ecological footprint. However, they suffer from slow kinetics and a comparatively low potential. In this paper, we have investigated alkali induced changes in the electronic structure of tetracyanoquinodimethane (TCNQ) to be used as cathode material in Li-ion batteries. Lithium was inserted stepwise into TCNQ thin films by exposure to lithium vapour and analysis by photoemission (PES) was performed. The evolution of core levels, electronic structure and Fermi-level with increasing lithium insertion into TCNQ was monitored. The results show that lithium insertion takes place under integer charge transfer and polaron formation. We find no indication of deterioration of the material. The consequences of evolution of electronic structure and polaron formation for electrode potential and kinetic properties of the material are discussed.


 Please wait while we load your content...
Please wait while we load your content...