Surface mediated chiral interactions between cyclodextrins and propranolol enantiomers: a SERS and DFT study†
Abstract
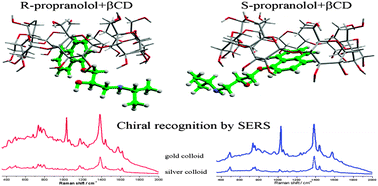
The nanoparticles mediated enantioselective recognition of propranolol enantiomers through native cyclodextrin complexation has been investigated by using surface-enhanced Raman spectroscopy (SERS). The highly efficient chiral recognition mechanism is based on a synergistic interaction between spherical noble metal nanoparticles, propranolol enantiomers and native cyclodextrins (CDs). Amongst the native cyclodextrins, β-CD has the highest chiral recognition ability for propranolol enantiomers, due to its specific shape and cavity size, thus producing the largest difference between the recorded SERS spectra of the two hosted enantiomers. The molecular interaction mechanism responsible for enantioselectivity was furthermore proven by quantum chemical calculations based on density functional theory (DFT). The theoretical calculations and experimental SER spectra allowed the assignment of functional moieties involved in the chiral recognition mechanism. The most important factors governing the highly efficient chiral probing by SERS are the fundamentally different mechanism of interaction between the R- and S-enantiomers and β-CD and the strength of interaction between the nanoparticle surface and the two propranolol–CD complexes. The role of metallic nanoparticles in the enantioselective recognition process has been experimentally evaluated by using silver and gold nanoparticles as SERS substrates, given their ability to interact differently with the complexes. The viability of this new method for chiral discrimination has been demonstrated for both substrates and could open new avenues for these kinds of applications.


 Please wait while we load your content...
Please wait while we load your content...