Open Access Article
Open Access ArticleGearing motion in cogwheel pairs of molecular rotors: weak-coupling limit†
Jiří
Kaleta
a,
Josef
Michl
ab,
Cécile
Mézière
c,
Sergey
Simonov
d,
Leokadiya
Zorina
d,
Pawel
Wzietek
e,
Antonio
Rodríguez-Fortea
f,
Enric
Canadell
g and
Patrick
Batail
*c
aInstitute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
bDepartment of Chemistry and Biochemistry, University of Colorado, Boulder, CO 80309-0215, USA
cLaboratoire MolTech-Anjou, Université d'Angers, CNRS UMR 6200, 2 Boulevard Lavoisier, 49045 Angers, France. E-mail: patrick.batail@univ-angers.fr
dInstitute of Solid State Physics RAS, 142432 Chernogolovka MD, Russia
eLaboratoire de Physique des Solides, Université de Paris-Sud, CNRS UMR 6502, Bâtiment 510, 91405 Orsay, France
fDepartament de Química Física i Inorgànica, Universitat Rovira i Virgili, Marcel·lí Domingo 1, 43007 Tarragona, Spain
gInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus de la UAB, 08193, Bellaterra, Spain
First published on 22nd September 2015
Abstract
Variable-temperature (VT) crystal structures, VT 1H spin–lattice relaxation in static crystals, and DFT modelling of the rotational barriers of BCP rotators in crystalline arrays of a rod-like molecule containing two 1,3-bis(ethynyl)bicyclo[1.1.1]pentane (BCP) units demonstrate that a correlated gearing motion occurs in the limit of a weak coupling between two rotors in a pair.
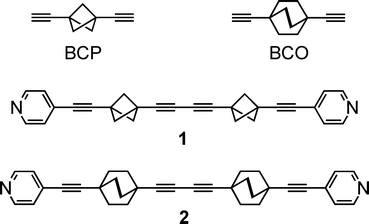
Understanding the mechanism of motion in crystalline arrays of molecular rotors with complex dynamics1 is a key step that will foster the development of molecular machines capable of performing useful work.2–8 The present investigation of the topology and dynamics of solid state assemblies of the rod-like molecule bis(3-(pyrid-4-ylethynyl)bicyclo[1.1.1]pent-1-yl)buta-1,3-diyne (1) that contains two 1,3-bis(ethynyl)bicyclo[1.1.1]pentane (BCP) rotators linked by a diyne fragment (Chart 1),9 was prompted (i) by our recent report10 of a correlated gearing motion in cogwheel pairs of a similar rod (2) with 1,4-bis(ethynyl)bicyclo[2.2.2]octane (BCO) rotators8 instead; and (ii) the demonstration that, in keeping with the one-dimensional topology of 2, a 4 Å shift of the rotor axles with respect to each other in the thermodynamic polymorph of 2 effectively suppresses the gearing motion.11 Another, yet different, example of correlated rotational motion in a pair is reported here for 1. The latter is shown to be a structural isomer of 2 where the BCP rotators do not rub against each other as much as the BCO rotators in the kinetic polymorph of 2, thereby defining a weak-coupling limit for the gearing motion in cogwheel pairs of molecular rotors.
Self-assembly by C–H⋯N hydrogen bonds in layers of parallel zig-zag strings of rods
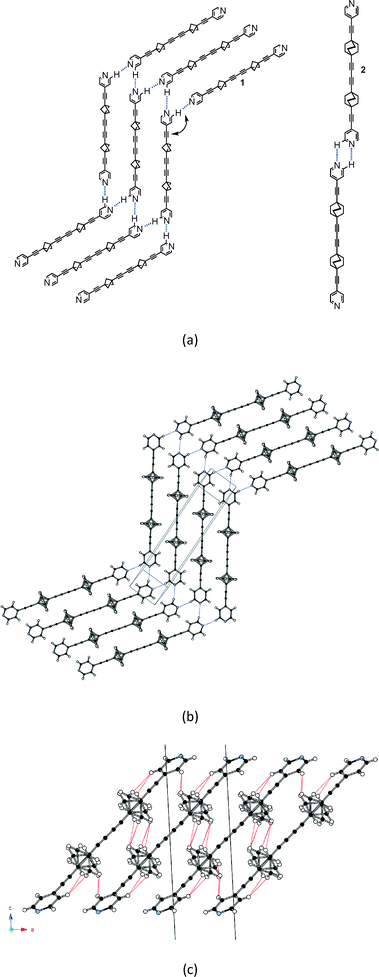
The molecular rod 1 was synthesized as reported earlier.9 Plate-like colourless crystals were obtained by slow cooling of an ethyl acetate solution and their structure was determined by X-ray diffraction at 293 K and 120 K.‡§As exemplified in Fig. 1, a striking feature distinguishes the patterns of self-assembly, and thus the topologies, of crystalline arrays of 1 and 2. Instead of two parallel C–H⋯N hydrogen bonds connecting successive rods in 2 into infinite one-dimensional strings, the same two hydrogen bonds (Table S1†) connect three rods in 1, creating infinite zig-zag strings with successive rod axles at an angle of 120° (Fig. 1a) and directing the formation of layers of parallel zig-zag strings of 1 (Fig. 1b). The latter are stacked at a canted angle along a (Fig. 1c).
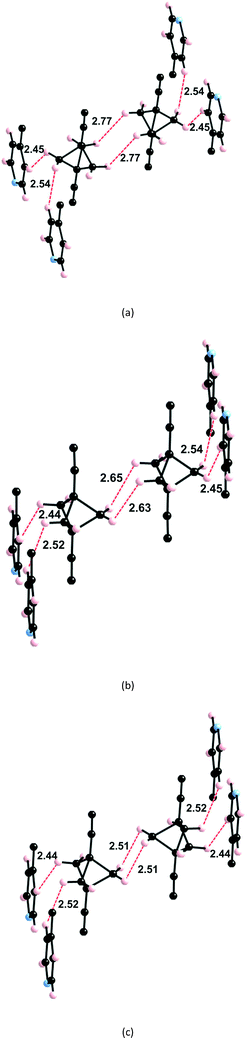
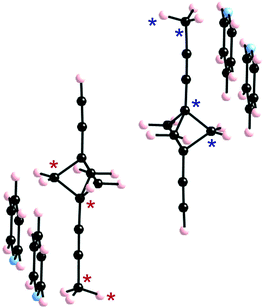
The BCP rotators are located at a single crystallographic site, distributed over two equilibrium positions whose occupancies are unbalanced (0.62 and 0.38 at 293 K; 0.66 and 0.34 at 120 K), but to a lesser extent than in the kinetic polymorph of 2 (0.88 and 0.12 at 293 K).10 The dotted red lines in Fig. 1c show the inventory of H⋯H interactions shorter than 2.8 Å. It is of interest to note that there is no such contact between BCP rotators in a layer (Fig. 1b). Instead, interacting BCP rotators in a pair are identified in the (a, c) plane as a result of the stacking of layers along a (Fig. 1c). The H⋯H distances associated with the three possible rotor–rotor interactions corresponding to the occupancies of the two equilibrium positions on a single site, i.e. Majority–Majority (2.770 Å (×2)), minority–minority (2.506 Å (×2)) and Majority–minority (2.630 and 2.650 Å), are shown in Fig. 2. In view of such data and our previous work on 2 (ref. 10) we anticipate the possibility of two different rotational barriers associated with: (i) a well-correlated synchronous motion of the two adjacent rotors and (ii) a higher energy asynchronous motion in which two blades of adjacent rotors rub against each other. However, there is an important difference between the two systems: whereas in 2 the H⋯H contacts in the minority–minority situation were as short as 2.1–2.2 Å, in 1 such H⋯H contacts are kept relatively long. Consequently, the two barriers should be relatively close in the present case.
Variable-temperature 1H spin–lattice relaxation identifies two relaxation processes
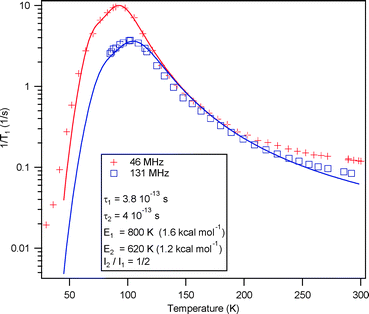
Variable-temperature (35–300 K) proton spin–lattice relaxation experiments were carried out at two fields (with 1H Larmor frequencies of 46 and 131 MHz) on a static crystalline sample, as described in earlier work.8,10–12 The correlation time τc is obtained by the fit of the 1H T1−1 data (Fig. 3) to the Kubo–Tomita formula, τc = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ea/kT), with two relaxation processes for the same spin temperature to yield rotational barriers of 1.23 kcal mol−1 = 620 K and 1.59 kcal mol−1 = 800 K, and τ0 of 4.0 × 10−13 s and 3.8 × 10−13 s, for the low and high energy processes, respectively. The occurrence of two relaxation processes for a rotator with two equilibrium positions with unbalanced occupancies on a single crystallographic site has been observed for the kinetic polymorph of 2 and attributed to the dynamic characteristics of a gearing motion within correlated cogwheel pairs.10 Note however that in 2, in agreement with the analysis above, the energy barriers are typically larger, especially for the high energy process (Ea = 6.1 kcal mol−1 = 3078 K).
exp(Ea/kT), with two relaxation processes for the same spin temperature to yield rotational barriers of 1.23 kcal mol−1 = 620 K and 1.59 kcal mol−1 = 800 K, and τ0 of 4.0 × 10−13 s and 3.8 × 10−13 s, for the low and high energy processes, respectively. The occurrence of two relaxation processes for a rotator with two equilibrium positions with unbalanced occupancies on a single crystallographic site has been observed for the kinetic polymorph of 2 and attributed to the dynamic characteristics of a gearing motion within correlated cogwheel pairs.10 Note however that in 2, in agreement with the analysis above, the energy barriers are typically larger, especially for the high energy process (Ea = 6.1 kcal mol−1 = 3078 K).
Modelling the energy barriers
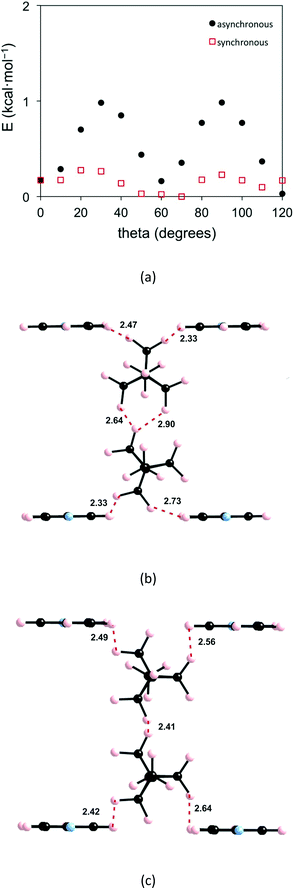
As discussed above (see Fig. 1c) the H atoms of a rotor in 1 are implicated in H⋯H contacts shorter than 2.8 Å with an adjacent rotor and two pyridine substituents. Consequently, the appropriate model to study the rotational motion in this solid is a couple of neighbouring rotors surrounded with four pyridine fragments, as shown in Fig. 4. This model was used in order to estimate the rotational barriers in the solid. The rotors have been modelled as a BCP unit (Chart 1) mimicking the situation in the solid whose triple bonds have been capped in the following manner: 1) by a H atom on the side of the ethynyl group; 2) by a H3C– on the side of the pyridine. The four neighbouring pyridines, which show relatively short contacts with the blades of the rotors, have been simply considered as pyridine molecules (Fig. 2). As in previous studies,8,10,11 density functional theory13 (DFT) calculations were carried out with the M06-2X functional14 using the Gaussian 09 code.15 Basis sets of the type 6-31-G(d,p) were used for C, N and H.16We have estimated the two rotational barriers for the synchronous and asynchronous motion of the two BCP rotators in a pair searching for the lowest-energy path by means of partial geometry optimizations. In our calculations, the outer H3C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– and –C
C– and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH groups of the two rotators are always kept fixed. For the synchronous motion, we fix the dihedral angle (θ1) defined by the atoms labelled with a red asterisk in Fig. 4 and change it every 10°. The coordinates for the two neighbouring rotators have been fully optimized within these restrictions. For the asynchronous motion, the dihedral angle defined by the atoms labelled with a blue asterisk in Fig. 4 were also fixed at the same value of the θ1 angle. The coordinates of the two rotators have been fully optimized within these restrictions. In both cases the four surrounding pyridine molecules were kept fixed at their crystallographic positions. We have verified that the associated H⋯H contacts never become too short and that this assumption is justified.
CH groups of the two rotators are always kept fixed. For the synchronous motion, we fix the dihedral angle (θ1) defined by the atoms labelled with a red asterisk in Fig. 4 and change it every 10°. The coordinates for the two neighbouring rotators have been fully optimized within these restrictions. For the asynchronous motion, the dihedral angle defined by the atoms labelled with a blue asterisk in Fig. 4 were also fixed at the same value of the θ1 angle. The coordinates of the two rotators have been fully optimized within these restrictions. In both cases the four surrounding pyridine molecules were kept fixed at their crystallographic positions. We have verified that the associated H⋯H contacts never become too short and that this assumption is justified.
The calculated barriers are reported in Fig. 5. That for the synchronous motion is very small, 0.28 kcal mol−1 = 141 K, whereas that for the asynchronous motion is larger yet not very high, 0.98 kcal mol−1 = 493 K. These values of the DFT barriers are about 1 kcal mol−1 lower than those obtained by the fit of the spin–lattice relaxation data. This is not unexpected in view of such small barriers, particularly since our discrete model does not take into account long-range contributions. The important result is that the energy difference between the two barriers is relatively small in both the spin–lattice relaxation experiments and the DFT calculations. These barriers mostly originate from the variation of the rotor–rotor H⋯H contacts. The optimized geometries for the higher energy structure of the two types of rotational motions are also shown in Fig. 5. It is clear that the rotor–rotor H⋯H contacts stay considerably long, which is the reason why the barriers are low, as expected. Thus, a picture emerges from this combined experimental and theoretical analysis of the motion of the rotors in a pair where adjacent rotors move gently in a synchronous, well correlated way as long as H⋯H contacts around 2.4 Å occurring in the asynchronous motion are avoided, which according to previous work10,11 is the onset for the development of repulsive, gear-slipping interactions.
Conclusions
We have shown that the rod-like molecule 1 containing two 1,3-bis(ethynyl)bicyclo[1.1.1]pentane (BCP) rotators, linked by a diyne fragment, self-assembles by C–H⋯N hydrogen bonds in a crystalline array that proved to be a structural isomer of the analogous rod, 2, carrying 1,4-bis(ethynyl)bicyclo[2.2.2]octane (BCO) rotators instead. As a result, the rod axles are shifted in 1 in such a way that the rotor–rotor interactions defining strong correlated cogwheel pairs in 2 are rather weakened, that is, the rotors do not rub against each other as much as in 1. Our investigation of the rotor dynamics by VT crystal structures and VT spin–lattice relaxation on static crystals, 1H T1−1, and DFT modelling of the rotational barriers reveals two low rotational barriers differing by 0.4–0.7 kcal mol−1, whose energies are rather lower that those in 2 (1.8 and 6.1 kcal mol−1), and concludes that the very same type of correlated gearing motion occurs at thermodynamic equilibrium yet in the limit of a weak coupling between two rotors in a pair, exemplified by a much smaller difference in energy between the low-energy gearing relaxation process and a higher-energy gear-slipping relaxation process.Acknowledgements
JK and JM gratefully acknowledge financial support from the European Research Council under the European Community's Framework Programme (FP7/2007-2013) ERC grant agreement no. 227756 and the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic (RVO: 61388963). This material is based upon the work supported by the National Science Foundation under Grant No. CHE-1265922. Work at Orsay was supported by the CNRS. Work at Angers was supported by the CNRS, the joint CNRS-Russian Federation grants PICS 6028 and RFBR-CNRS 12-03-91059 (Chernogolovka), and by the Région des Pays de la Loire Grant MOVAMOL. Work in Bellaterra and Tarragona was supported by the Spanish Ministerio de Economía y Competitividad (Projects FIS2012-37549-C05-05 and CTQ2014-52774-P) and Generalitat de Catalunya (2014GR301 and 2014GR199).Notes and references
- B. Rodríguez-Molina, S. Peŕez-Estrada and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2013, 135, 10388 CrossRef CAS PubMed; B. Rodríguez-Molina, N. Farfán, M. Romero, J. M. Méndez-Stivalet, R. Santillan and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2011, 133, 7280 CrossRef PubMed; M. A. Garcia-Garibay, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10771 CrossRef PubMed; T.-A. V. Khuong, J. E. Nunez, C. E. Godinez and M. A. Garcia-Garibay, Acc. Chem. Res., 2006, 39, 413 CrossRef PubMed; S. D. Karlen, H. Reyes, R. E. Taylor, S. I. Khan, M. F. Hawthorne and M. A. Garcia-Garibay, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14973 CrossRef PubMed; V. Vogelsberg and M. A. Garcia-Garibay, Chem. Soc. Rev., 2012, 41, 1892 RSC.
- B. L. Feringa, Acc. Chem. Res., 2001, 34, 504 CrossRef CAS PubMed; D. Horinek and J. Michl, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 14175 CrossRef PubMed; G. S. Kottas, L. I. Clarke, D. Horinek and J. Michl, Chem. Rev., 2005, 105, 1281 CrossRef PubMed; E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72 CrossRef PubMed; K. Skopek, M. C. Hershberger and J. A. Gladysz, Coord. Chem. Rev., 2007, 251, 1723 CrossRef PubMed; L. Kobr, K. Zhao, Y. Shen, R. K. Shoemaker, C. T. Rogers and J. Michl, Cryst. Growth Des., 2014, 14, 559 Search PubMed.
- W. Setaka and K. Yamaguchi, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9271 CrossRef CAS PubMed; W. Setaka and K. Yamaguchi, J. Am. Chem. Soc., 2012, 134, 17932 CrossRef PubMed.
- W. Zhang and R.-G. Xiong, Chem. Rev., 2012, 112, 1163 CrossRef CAS PubMed; W. Zhang, H.-Y. Ye, R. Graf, H. W. Spiess, Y.-F. Yao, R.-Q. Zhu and R.-G. Xiong, J. Am. Chem. Soc., 2013, 135, 5230 CrossRef PubMed.
- S. Horiuchi, Y. Tokunaga, G. Giovanetti, S. Picozzi, H. Itoh, R. Shimano, R. Kumai and Y. Tokura, Nature, 2010, 463, 789 CrossRef CAS PubMed; T. Akutagawa, H. Koshinaka, D. Sato, S. Takeda, S. I. Noro, H. Takahashi, R. Kumai, Y. Tokura and T. Nakamura, Nat. Mater., 2009, 8, 342 CrossRef PubMed.
- S. Yamamoto, H. Iida and E. Yashima, Angew. Chem., Int. Ed., 2013, 52, 6849 CrossRef CAS PubMed.
- Q.-C. Zhang, F.-T. Wu, H.-M. Hao, H. Xu, H.-X. Zhao, L.-S. Long, R.-B. Huang and L.-S. Zheng, Angew. Chem., Int. Ed., 2013, 52, 12602 CrossRef CAS PubMed.
- C. Lemouchi, H. M. Yamamoto, R. Kato, S. Simonov, L. Zorina, A. Rodriguez-Fortea, E. Canadell, P. Wzietek, K. Iliopoulos, D. Gindre, M. Chrysos and P. Batail, Cryst. Growth Des., 2014, 14, 3375 Search PubMed; C. Lemouchi, C. S. Vogelsberg, S. Simonov, L. Zorina, P. Batail, S. Brown and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2011, 133, 6371 CrossRef CAS PubMed.
- J. Kaleta, P. I. Dron, K. Zhao, Y. Shen, I. Císařová, C. T. Rogers and J. Michl, J. Org. Chem., 2015, 80, 6173 CrossRef CAS PubMed; M. Cipolloni, J. Kaleta, M. Mašát, P. I. Dron, Y. Shen, K. Zhao, C. T. Rogers, R. K. Shoemaker and J. Michl, J. Phys. Chem. C, 2015, 119, 8805 Search PubMed; J. Kaleta, M. Nečas and C. Mazal, Eur. J. Org. Chem., 2012, 25, 4783 CrossRef PubMed; J. Kaleta, J. Michl and C. Mazal, J. Org. Chem., 2010, 75, 2350 CrossRef PubMed; C. Mazal, O. Škarka, J. Kaleta and J. Michl, Org. Lett., 2006, 8, 749 CrossRef PubMed; P. F. H. Schwab, B. C. Noll and J. Michl, J. Org. Chem., 2002, 67, 5476 CrossRef PubMed; M. D. Levin, P. Kaszynski and J. Michl, Chem. Rev., 2000, 100, 169 CrossRef PubMed; P. Kaszynski and J. Michl, [n]Staffanes, in Advances in Strain in Organic Chemistry, IV, ed. B. Halton, JAI Press Inc., Greenwich, CT, 1995, p. 283 CrossRef; M. D. Levin, S. J. Hamrock, P. Kaszynski, A. B. Shtarev, G. A. Levina, B. C. Noll, M. E. Ashley, R. Newmark, G. G. I. Moore and J. Michl, J. Am. Chem. Soc., 1997, 119, 12750 CrossRef; A. B. Shtarev, E. Pinkhassik, M. D. Levin, I. Stibor and J. Michl, J. Am. Chem. Soc., 2001, 123, 3484 CrossRef PubMed.
- C. Lemouchi, K. Iliopoulos, L. Zorina, S. Simonov, P. Wzietek, T. Cauchy, A. Rodríguez-Fortea, E. Canadell, J. Kaleta, J. Michl, D. Gindre, M. Chrysos and P. Batail, J. Am. Chem. Soc., 2013, 135, 9366 CrossRef CAS PubMed.
- G. Bastien, C. Lemouchi, M. Allain, P. Wzietek, A. Rodríguez-Fortea, E. Canadell, K. Iliopoulos, D. Gindre, M. Chrysos and P. Batail, CrystEngComm, 2014, 16, 1241 RSC.
- See also: A. Comotti, S. Bracco, T. Ben, S. Qiu and P. Sozzani, Angew. Chem., Int. Ed., 2014, 53, 1043 CrossRef CAS PubMed; A. Comotti, S. Bracco, A. Yamamoto, M. Beretta, T. Hirukawa, N. Tohnai, M. Miyata and P. Sozzani, J. Am. Chem. Soc., 2014, 136, 618 CrossRef PubMed.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864 CrossRef; W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133 CrossRef.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B1, Gaussian Inc., Wallingford CT, 2009 Search PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef CAS PubMed.
- A. J. M. Duisenberg, L. M. J. Kroon-Batenburg and A. M. M. Schreurs, J. Appl. Crystallogr., 2003, 36, 220 CrossRef CAS; CrysAlisPro, Version 1.171.37.34c, Agilent Technologies Search PubMed.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany, 1996 Search PubMed; SCALE3ABSPACK, Version 1.171.37.34c, Agilent Technologies Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Table S1. CCDC 1412010–1412011. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ce01372k |
| ‡ Crystallography. Single crystal X-ray diffraction data were collected with MoKα (0.71073 Å) radiation on a Bruker Kappa CCD diffractometer at room temperature and with an Oxford Diffraction Gemini-R diffractometer at 120 K (in a cooled nitrogen gas stream). The 293 and 120 K data were processed using the EvalCCD and CrysAlisPro software,17 respectively; empirical absorption correction was applied with the programs SADABS and Scale3AbsPack.18 The structure was solved by direct methods followed by Fourier syntheses and refined by the full-matrix least-squares method in an anisotropic approximation for all non-hydrogen atoms using the program SHELX-97.19 H atoms were refined in a riding model with Uiso(H) = 1.2 Ueq(C). The small thickness of the plate-type crystal and twinning resulted in rather high values of the reliability factors after the structure refinement. |
| § Crystal data for 1 at 293 K: C28H20N2, M = 384.46, monoclinic P21/c, a = 5.7123(19), b = 5.7076(17), c = 33.016(18) Å, β = 93.68(5)°, V = 1074.2(8) Å3, Z = 2, μ = 0.70 cm−1, 2Θmax = 54.2°, 7162 reflections measured, 2400 unique (Rint = 0.165), 790 with I > 2σ(I), 166 parameters refined, R(F2) = 0.0769, wR(F2) = 0.1598, GOF = 0.861. At 120 K: monoclinic P21/c, a = 5.6780(10), b = 5.6456(7), c = 32.491(12) Å, β = 93.09(2)°, V = 1040.0(4) Å3, Z = 2, μ = 0.72 cm−1, 2Θmax = 56.7°, 4203 reflections measured, 2332 unique (Rint = 0.0617), 1635 with I > 2σ(I), 165 parameters refined, R(F2) = 0.0954, wR(F2) = 0.2314, GOF = 1.020. |
| This journal is © The Royal Society of Chemistry 2015 |