Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Manipulating connecting nodes through remote alkoxy chain variation in coordination networks with 4′-alkoxy-4,2′:6′,4′′-terpyridine linkers†
Y. Maximilian
Klein
,
Alessandro
Prescimone
,
Edwin C.
Constable
and
Catherine E.
Housecroft
*
Department of Chemistry, University of Basel, Spitalstrasse 51, CH-4056 Basel, Switzerland. E-mail: catherine.housecroft@unibas.ch
First published on 6th July 2015
Abstract
The effects of increasing the length of the alkoxy substituent in 4′-alkoxy-4,2′:6′,4′′-terpyridines when they are combined with cadmium(II) nitrate under conditions of room temperature crystallization and in the same cadmium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand (1
ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) ratio have been investigated. The divergent ligand 4′-n-propoxy-4,2′:6′,4′′-terpyridine (2) reacts with Cd(NO3)2·4H2O to give [{Cd2(NO3)4(2)3}·3CHCl3]n in which the Cd atoms act as 3-connecting nodes and assemble into a (6,3) net with each ligand 2 linking adjacent Cd atoms. One of the three independent n-propoxy groups nestles into a cleft in the next 2-dimensional sheet; this ‘tail-in-pocket’ interaction restricts the length of the alkyl chain that can be accommodated. Replacing the n-propoxy by an n-pentoxy, n-hexoxy or n-heptoxy substituent results in a switch from a (6,3) to (4,4) net; in [{Cd2(NO3)4(3)4}·3CHCl3]n (3 = 4′-n-pentoxy-4,2′:6′,4′′-terpyridine) and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n (4 = 4′-n-hexoxy-4,2′:6′,4′′-terpyridine), each Cd atom is a 4-connecting node with trans-nitrato ligands, while in [{Cd(NO3)2(5)2}·2MeOH]n (5 = 4′-n-heptoxy-4,2′:6′,4′′-terpyridine) a cis-arrangement of nitrato ligands is observed. The reaction between Cd(NO3)2·4H2O and 4 was also investigated using a 1
3) ratio have been investigated. The divergent ligand 4′-n-propoxy-4,2′:6′,4′′-terpyridine (2) reacts with Cd(NO3)2·4H2O to give [{Cd2(NO3)4(2)3}·3CHCl3]n in which the Cd atoms act as 3-connecting nodes and assemble into a (6,3) net with each ligand 2 linking adjacent Cd atoms. One of the three independent n-propoxy groups nestles into a cleft in the next 2-dimensional sheet; this ‘tail-in-pocket’ interaction restricts the length of the alkyl chain that can be accommodated. Replacing the n-propoxy by an n-pentoxy, n-hexoxy or n-heptoxy substituent results in a switch from a (6,3) to (4,4) net; in [{Cd2(NO3)4(3)4}·3CHCl3]n (3 = 4′-n-pentoxy-4,2′:6′,4′′-terpyridine) and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n (4 = 4′-n-hexoxy-4,2′:6′,4′′-terpyridine), each Cd atom is a 4-connecting node with trans-nitrato ligands, while in [{Cd(NO3)2(5)2}·2MeOH]n (5 = 4′-n-heptoxy-4,2′:6′,4′′-terpyridine) a cis-arrangement of nitrato ligands is observed. The reaction between Cd(NO3)2·4H2O and 4 was also investigated using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of reagents; this leads to the assembly of the 1-dimensional ladder [Cd2(NO3)4(MeOH)(4)3]n in which each Cd atom is a 3-connecting node. In each structure, face-to-face π-stacking of the central pyridine rings or of pyridine/phenyl rings of ligands in adjacent sheets or chains is a primary packing interaction; the role of van der Waals interactions as the chain length increases is discussed. Powder diffraction confirmed that each coordination polymer or network characterized by single crystal X-ray crystallography was representative of the bulk sample. The solid-state emission properties of ligands 2, 3 and 4 and their coordination polymers are reported; the blue emission of the free ligands is red-shifted by up to 59 nm upon formation of the coordination networks, and quantum yields are in the range 11–22%.
1 ratio of reagents; this leads to the assembly of the 1-dimensional ladder [Cd2(NO3)4(MeOH)(4)3]n in which each Cd atom is a 3-connecting node. In each structure, face-to-face π-stacking of the central pyridine rings or of pyridine/phenyl rings of ligands in adjacent sheets or chains is a primary packing interaction; the role of van der Waals interactions as the chain length increases is discussed. Powder diffraction confirmed that each coordination polymer or network characterized by single crystal X-ray crystallography was representative of the bulk sample. The solid-state emission properties of ligands 2, 3 and 4 and their coordination polymers are reported; the blue emission of the free ligands is red-shifted by up to 59 nm upon formation of the coordination networks, and quantum yields are in the range 11–22%.
Introduction
Diversity in the architectures of 2- and 3-dimensional coordination polymers is engineered through the use of complementary metal nodes and organic (ligand) linkers.1–7 Oligopyridines remain popular tectons in supramolecular assemblies,8,9 and over the last 15 years, the divergent 4,2′:6′,4′′-terpyridine (4,2′:6′,4′′-tpy) domain has proved to be a versatile linker10 binding metal ions through the outer pyridine rings (Scheme 1). 4,2′:6′,4′′-Terpyridines are easily functionalized in the 4′-position to incorporate additional coordination sites11 or sterically variable substituents (e.g. bulky12 or long chain,13,14 alkyl groups) that influence packing interactions and assembly motifs.Although strategies towards 2- and 3-dimensional networks are currently being developed using ditopic 4,2′:6′,4′′-tpy and its isomeric 3,2′:6′,3′′-tpy linkers,15,16 the coordination chemistry of 4,2′:6′,4′′-tpy remains dominated by 1-dimensional chains. This preference can be modified by turning attention to the metal node. For example, linear {Zn2(OAc)4} nodes (Scheme 1) combined with 4,2′:6′,4′′-tpys result in flat, zig-zag chains, whereas non-linear nodes (often incorporating zinc(II) halides) lead to helical polymers.10 Single chains can be extended into double-stranded chains17 by the use of {Cd2(OAc)4} nodes which are structurally distinct from their zinc(II) counterparts (Scheme 2). This design principle is extended using {Mn3(OAc)6} nodes for the assembly of triple-stranded chains,18 but unexpectedly, planar {Zn5(OAc)10} nodes lead to quadruple-stranded, rather than pentuple, chains.19 Double chains in this family possess a ladder topology, with the rungs of the ladder defined by the {Cd2(OAc)4}-bridges (Scheme 2). The bridging mode of the acetato ligand is key to the formation of {Mn(OAc)2n} nodes, and the use of [AcO]− to assist the formation of multimetal, in particular zinc, cluster building blocks is well established with [Zn4(μ-OAc)6(μ4-O)]20 derivatives being fundamental to many metal organic frameworks (MOFs).21
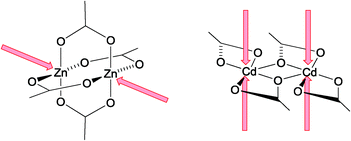 | ||
| Scheme 2 Structurally different {Zn2(OAc)4} and {Cd2(OAc)4} nodes combine with 4,2′:6′,4′′-tpy to give single and double-stranded chains, respectively. | ||
Within the coordination chemistry of 4,2′:6′,4′′-terpyridines, a switch from cadmium(II) acetate to nitrate significantly influences the coordination polymer assembly. For example, 4′-phenyl-4,2′:6′,4′′-tpy reacts with Cd(NO3)2·4H2O to give a 2-dimensional (4,4) net,22 while introducing 4-MeOC6H4,23 4-MeSC6H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 or 4-HC
24 or 4-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4
CC6H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 units into the 4′-position of 4,2′:6′,4-tpy results in ladder assemblies in which each rung is defined by a 4,2′:6′,4′′-tpy ligand. However, these ladders with a 2
24 units into the 4′-position of 4,2′:6′,4-tpy results in ladder assemblies in which each rung is defined by a 4,2′:6′,4′′-tpy ligand. However, these ladders with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of Cd
3 ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand have been produced using different ratios of precursors in the crystallization experiments. We present here a systematic investigation of reactions of 4′-(4-alkoxyphenyl)-4,2′:6′,4′′-terpyridines (Scheme 3) with cadmium(II) nitrate under room temperature conditions of crystallization. Our focus is to use a constant input ratio of Cd(NO3)2·4H2O to ligand (1
ligand have been produced using different ratios of precursors in the crystallization experiments. We present here a systematic investigation of reactions of 4′-(4-alkoxyphenyl)-4,2′:6′,4′′-terpyridines (Scheme 3) with cadmium(II) nitrate under room temperature conditions of crystallization. Our focus is to use a constant input ratio of Cd(NO3)2·4H2O to ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in order to determine preferred assemblies and their reproducibility. We then demonstrate the effects of reducing the amount of ligand (Cd
3) in order to determine preferred assemblies and their reproducibility. We then demonstrate the effects of reducing the amount of ligand (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand = 1
ligand = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the assembly process.
1) on the assembly process.
Experimental
Ligands 1–5 were prepared as previously described.14Crystallography
Crystallographic data were collected on a Bruker-Nonius Kappa APEX diffractometer; data reduction, solution and refinement used APEX225 and CRYSTALS.26 Powder diffraction data were collected on a Stoe Stadi P powder diffractometer. Structural diagrams and structural analysis were carried out using Mercury v. 3.5.1,27,28 and TOPOS.29 The solvent region of three of the structures treated with the program SQUEEZE,30 and the electron density removed was equated to appropriate solvent molecules and added to relevant formulae (see below). High thermal motion in some of the alkyl chains meant that some carbon atoms had to be refined isotropically; some bond parameters in these chains were restrained to chemically reasonable values.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 reflections, 14
690 reflections, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 unique, Rint = 0.090. Refinement of 14
425 unique, Rint = 0.090. Refinement of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 reflections (854 parameters) with I > 2σ(I) converged at final R1 = 0.0814 (R1 all data = 0.1228), wR2 = 0.2242 (wR2 all data = 0.2753), gof = 0.9871. CCDC 1402483.
419 reflections (854 parameters) with I > 2σ(I) converged at final R1 = 0.0814 (R1 all data = 0.1228), wR2 = 0.2242 (wR2 all data = 0.2753), gof = 0.9871. CCDC 1402483.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174.7(17) Å3, Z = 4, Dc = 1.43 Mg m−3, μ(Cu-Kα) = 5.601 mm−1, T = 123 K. Total 76
174.7(17) Å3, Z = 4, Dc = 1.43 Mg m−3, μ(Cu-Kα) = 5.601 mm−1, T = 123 K. Total 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477 reflections, 19
477 reflections, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 unique, Rint = 0.047. Refinement of 19
048 unique, Rint = 0.047. Refinement of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 reflections (1232 parameters) with I > 2σ(I) converged at final R1 = 0.1340 (R1 all data = 0.1419), wR2 = 0.3465 (wR2 all data = 0.3513), gof = 1.0024. CCDC 1402485.
027 reflections (1232 parameters) with I > 2σ(I) converged at final R1 = 0.1340 (R1 all data = 0.1419), wR2 = 0.3465 (wR2 all data = 0.3513), gof = 1.0024. CCDC 1402485.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631.6(9) Å3, Z = 4, Dc = 1.41 Mg m−3, μ(Cu-Kα) = 4.500 mm−1, T = 123 K. Total 44
631.6(9) Å3, Z = 4, Dc = 1.41 Mg m−3, μ(Cu-Kα) = 4.500 mm−1, T = 123 K. Total 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477 reflections, 18
477 reflections, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 unique, Rint = 0.089. Refinement of 11
427 unique, Rint = 0.089. Refinement of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 009 reflections (1191 parameters) with I > 2σ(I) converged at final R1 = 0.1026 (R1 all data = 0.1279), wR2 = 0.2838 (wR2 all data = 0.3071), gof = 1.0024. CCDC 1402486.
009 reflections (1191 parameters) with I > 2σ(I) converged at final R1 = 0.1026 (R1 all data = 0.1279), wR2 = 0.2838 (wR2 all data = 0.3071), gof = 1.0024. CCDC 1402486.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3924 reflections, 9826 unique, Rint = 0.087. Refinement of 5855 reflections (659 parameters) with I > 2σ(I) converged at final R1 = 0.0986 (R1 all data = 0.1359), wR2 = 0.2752 (wR2 all data = 0.3369), gof = 0.9707. CCDC 1402484.
3924 reflections, 9826 unique, Rint = 0.087. Refinement of 5855 reflections (659 parameters) with I > 2σ(I) converged at final R1 = 0.0986 (R1 all data = 0.1359), wR2 = 0.2752 (wR2 all data = 0.3369), gof = 0.9707. CCDC 1402484.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.1304(7), b = 16.5224(9), c = 19.2845(10) Å, α = 83.670(3), β = 82.840(3), γ = 70.242(3)°, U = 3896.3(2) Å3, Z = 2, Dc = 1.477 Mg m−3, μ(Cu-Kα) = 5.007 mm−1, T = 123 K. Total 49
, a = 13.1304(7), b = 16.5224(9), c = 19.2845(10) Å, α = 83.670(3), β = 82.840(3), γ = 70.242(3)°, U = 3896.3(2) Å3, Z = 2, Dc = 1.477 Mg m−3, μ(Cu-Kα) = 5.007 mm−1, T = 123 K. Total 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 reflections, 13
970 reflections, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 661 unique, Rint = 0.039. Refinement of 12
661 unique, Rint = 0.039. Refinement of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 221 reflections (1018 parameters) with I > 2σ(I) converged at final R1 = 0.0540 (R1 all data = 0.0586), wR2 = 0.1212 (wR2 all data = 0.1228), gof = 0.9870. CCDC 1402482.
221 reflections (1018 parameters) with I > 2σ(I) converged at final R1 = 0.0540 (R1 all data = 0.0586), wR2 = 0.1212 (wR2 all data = 0.1228), gof = 0.9870. CCDC 1402482.
Results and discussion
n-Propoxy-tailed 4,2′:6′,4′′-terpyridine
We recently reported that under crystallization conditions in MeOH/CHCl3, ligand 1 (Scheme 3) reacts with Cd(NO3)2·4H2O to give a 1-dimensional coordination ladder [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n.23 The ladder possesses a Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 2
ligand ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and both the rungs and rails of the ladder are defined by bridging ligands (Fig. 1). In order to probe the effects of lengthening the alkoxy chain in the linker, crystallization experiments combining Cd(NO3)2·4H2O with ligands 2, 3, 4 or 5 with ratios of Cd
3, and both the rungs and rails of the ladder are defined by bridging ligands (Fig. 1). In order to probe the effects of lengthening the alkoxy chain in the linker, crystallization experiments combining Cd(NO3)2·4H2O with ligands 2, 3, 4 or 5 with ratios of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand of 1
ligand of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in MeOH/CHCl3 were conducted. These resulted in the growth of X-ray quality crystals.
3 in MeOH/CHCl3 were conducted. These resulted in the growth of X-ray quality crystals.
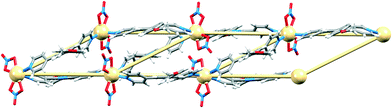 | ||
| Fig. 1 Superimposition of the structure and TOPOS representation of part of one ladder in [{Cd2(1)3(NO3)4}·CHCl3·MeOH]n.23 | ||
Structural analysis of a crystal selected from the bulk sample of crystals grown from the reaction of 2 (n-propoxy-tailed ligand) and Cd(NO3)2·4H2O confirmed the formation of [{Cd2(NO3)4(2)3}·3CHCl3]n. Cell checks on other crystals from the batch revealed consistent cell parameters. A comparison of the powder diffraction pattern for a batch of ground crystals from the bulk sample, with that of predicted from the single crystal structure is shown in Fig. S1.† The data are consistent with the single crystal being representative of the bulk sample.
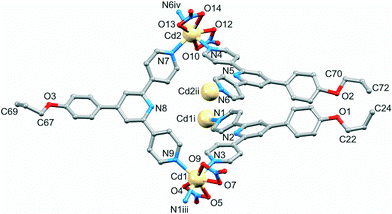
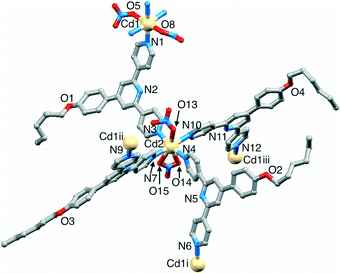
[{Cd2(NO3)4(2)3}·3CHCl3]n crystallizes in the monoclinic space group P21/n, and the asymmetric unit contains two independent Cd atoms and three independent ligands (Fig. 2). Although the Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 2
ligand ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 is the same as in the coordination ladder [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n, propagation of the structure in [{Cd2(NO3)4(2)3}·3CHCl3]n leads to a 2-dimensional (6,3) net which lies in the bc-plane. Each of Cd1 and Cd2 is 7-coordinate, and is bound by the outer pyridine ring of three different ligands 2 and two bidentate nitrato ligands. Bond lengths are unexceptional (see caption to Fig. 2) and the bite angles of the four independent nitrato ligands are in the range 51.1(3)–52.2(3)°. The three N-donors at each Cd centre define a T-shaped environment with N–Cd–N angles (N1iii–Cd1–N9 = 103.5(3) and N3–Cd1–N9 = 91.7(3)°, N6iv–Cd2–N7 = 104.7(3) and N4–Cd2–N7 = 91.6(3)°).
3 is the same as in the coordination ladder [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n, propagation of the structure in [{Cd2(NO3)4(2)3}·3CHCl3]n leads to a 2-dimensional (6,3) net which lies in the bc-plane. Each of Cd1 and Cd2 is 7-coordinate, and is bound by the outer pyridine ring of three different ligands 2 and two bidentate nitrato ligands. Bond lengths are unexceptional (see caption to Fig. 2) and the bite angles of the four independent nitrato ligands are in the range 51.1(3)–52.2(3)°. The three N-donors at each Cd centre define a T-shaped environment with N–Cd–N angles (N1iii–Cd1–N9 = 103.5(3) and N3–Cd1–N9 = 91.7(3)°, N6iv–Cd2–N7 = 104.7(3) and N4–Cd2–N7 = 91.6(3)°).
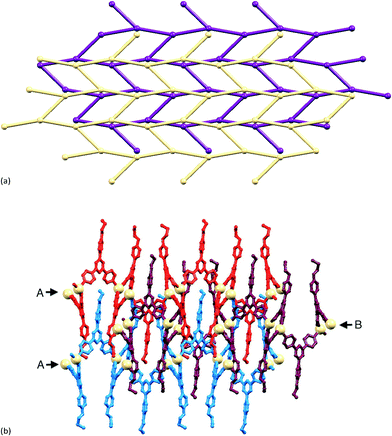
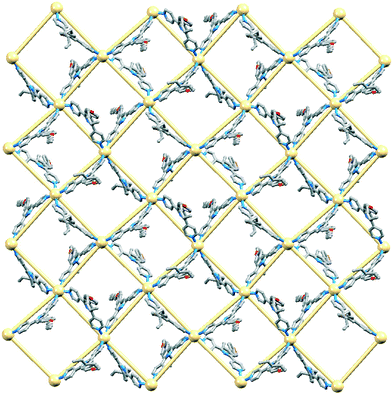
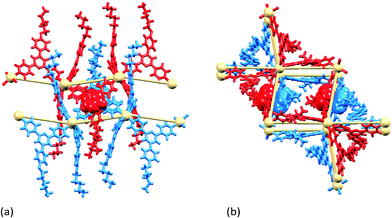
Around each 6-membered ring in the (6,3) net, the bridging ligands with extended chains are in an up/up/down/up/up/down sequence, and the nets stack over one another in an ABAB⋯ arrangement with A and B layers related by inversion (Fig. 3a). The closest Cd⋯Cd separation between nets is 7.836(1) Å, which is significantly shorter that the separations of 12.3597(9) to 12.9881(9) Å within each hexacycle in the (6,3) net; the latter distances are imposed by the span of bridging ligand 2. Fig. 3b illustrates the penetration of the 4-propoxyphenyl groups through adjacent sheets. The chain containing atom O3 is neatly accommodated (Fig. 4) within a pocket comprising three ligands 2 containing N3v, N5v and N8v (symmetry code v = 1 + x, y, z). The pyridine ring with N8 is sandwiched between two phenyl rings in adjacent chains; angles between the least squares planes of the rings in the triple π-stack (Fig. 4) are 7.9 and 6.2°, and the phenylcentroid⋯pyridineplane distances are 3.54 and 3.53 Å. The close H⋯H contacts highlighted in green in Fig. 4 are an important feature of this ‘tail-in-pocket’ interaction, and limit the length of the alkyl chain that can be accommodated. We were able to obtain preliminary structural data on crystals grown from the reaction of Cd(NO3)2·4H2O and 4′-(4-n-butoxyphenyl)-4,2′:6′,4′′-tpy. Significantly, the cell dimensions (a = 15.241(9), b = 25.2808(13), c = 21.913(2) Å, β = 90.75(4)°) are similar to those of [{Cd2(NO3)4(2)3}·3CHCl3]n and the preliminary determination confirmed the assembly of a (6,3) with analogous structural features to those shown in Fig. 3 and 4. This implies that no major structural perturbation occurs on going from an n-propoxy to n-butoxy functionalized ligand.
From n-propoxy to n-pentoxy and n-hexoxy tails
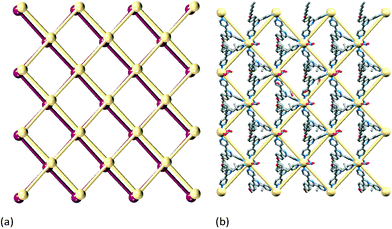
Reaction of Cd(NO3)2·4H2O with 3 or 4 (n-pentoxy or n-hexoxy derivatives, Scheme 3) leads to [{Cd2(NO3)4(3)4}·3CHCl3]n or [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, respectively. The compounds are structurally analogous, crystallizing in the space group P21/c with similar cell dimensions. Cell parameters consistent with those in the experimental section for [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n were obtained for represenative crystals chosen from the bulk sample. Fig. S2 and S3† compare the powder diffraction patterns predicted from the single crystal structures of the two coordination polymers with those of ground crystals from the bulk material, and with experimental powder patterns for the precursors. The data confirm that the single crystals chosen were representative of the bulk samples.In the light of the restricted space associated with the ‘tail-in-pocket’ interaction in [{Cd2(NO3)4(2)3}·3CHCl3]n and its n-butoxy analogue, a change in structure on going to the longer n-pentoxy or n-hexoxy chains is not unexpected. Fig. 5 shows the asymmetric unit (with symmetry generated atoms) in [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, with two independent Cd atoms and four independent ligands 4. Likewise, [{Cd2(NO3)4(3)4}·3CHCl3]n contains two independent Cd atoms and four independent molecules of 3. The Cd atoms are octahedrally sited, coordinated by four ligands and two, mutually trans nitrato ligands. The Cd–N bond lengths are in the range 2.317(9) to 2.362(8) Å in [{Cd2(NO3)4(3)4}·3CHCl3]n, and 2.286(8) to 2.407(4) Å in [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n. The coordination modes of the nitrato ligands in the two compounds are monodentate, bidentate (Fig. 5) or monodentate with an additional long contact; Cd–O distances range from 2.360(7) to 2.879(9) Å in [{Cd2(NO3)4(3)4}·3CHCl3]n and from 2.310(12) to 2.616(8) Å in [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n. In each structure, one nitrato ligand is disordered and has been modelled over two sites with fractional occupancies of 0.60/0.40 or 0.65/0.35. Each Cd atom in [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n acts as a 4-connecting node giving the (4,4) coordination networks shown in Fig. 6 and 7. Each rhombus in the net in [{Cd2(NO3)4(3)4}·3CHCl3]n (Fig. 6a) is characterized by Cd⋯Cd distances in a range 12.502(3) to 13.063(3) Å and internal angles of 79.02(1), 99.89(1), 79.91(1) and 101.19(1)°. In the net in [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n (Fig. 7), each rhombus has Cd⋯Cd distances between 12.4678(9) and 12.771(1) Å and internal angles of 86.26(1), 92.31(1), 87.61(1) and 93.82(1)°. The bridging ligands 3 or 4 linking the Cd nodes project above and below the net (Fig. 6b), and adopt an up/up/up/down arrangement working around the four sides of the rhombus.
In [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, the (4,4) nets lie parallel to the ab-plane. The alkoxy chains are in extended or close to extended conformations and are directed approximately along the c-axis (Fig. 6b). This has the effect of locking the nets with the 4-membered macrocycles almost directly over one another as shown in Fig. 8 for [{Cd2(NO3)4(3)4}·3CHCl3]n. Pairs of pyridine rings (containing N2 and N5iv, symmetry code iv = x, 1/2 − y, −1/2 + z) in ligands from adjacent sheets engage in efficient face-to-face π-stacking interactions; the angle between the least squares planes of the rings is 1.5° and ring-plane to centroid separation = 3.38 Å. (These parameters for the analogous interactions in [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n are 0.4° and 3.49 Å.) These interactions (two per macrocycle, Fig. 8b) are central to interlocking the sheets. The shortest Cd⋯Cd separations between sheets are 8.940(2) and 8.894(2) Å in [{Cd2(NO3)4(3)4}·3CHCl3]n, and 8.468(1) and 8.433(1) Å in [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n; this places the 4′-phenyl substituents of ligands 3 or 4 within the (4,4) net defined by the Cd atoms (central part of Fig. 8a). Additional voids in the lattice are occupied by solvent molecules. SQUEEZE30 was used to treat the solvent region; the rationalized solvent content in the structurally similar coordination networks in [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n is consistent with the greater spatial demands of ligand 4versus3.
n-Hexoxy to n-heptoxy tails and a switch from trans- to cis-coordination
The effects of further chain lengthening were investigated by treating Cd(NO3)2·4H2O with ligand 5 which contains an n-heptoxy tail (Scheme 3). X-ray quality crystals grown under the same conditions as those with ligands 2–4 proved to be [{Cd(NO3)2(5)2}·2MeOH]n. The powder diffraction data shown in Fig. S4† are consistent with the single crystal being representative of the bulk sample.As in the (4,4) networks with ligands 3 and 4, the Cd atom in [{Cd(NO3)2(5)2}·2MeOH]n is octahedrally sited and is coordinated by four 4,2′:6′,4′′-tpy and two nitrato ligands. However, the nitrato groups are mutually cis (Fig. 9a), in contrast to trans-arrangements in [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n. Bond parameters for the coordination sphere are given in the caption to Fig. 9a. The alkoxy chain with O1 is in a fully extended conformation while that with O2 is folded out of the plane of the phenyl ring to which it is bonded. The Cd atoms act as 4-connecting nodes and assemble into a (4,4) net. Compared to the up/up/up/down arrangement of bridging ligands around each 4-membered metallomacrocycle in [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, those in [{Cd(NO3)2(5)2}·2MeOH]n adopt an up/down/up/down arrangement (Fig. 9b). The two independent Cd⋯Cd distances within the (4,4) net are 12.938(2) and 12.630(2) Å. The Cd nodes in each net lie in a plane (deviation is ±0.1 Å); the sheets stack over one another (Fig. 10a) and the shortest Cd⋯Cd separations are 8.566(2) and 8.950(2) Å.
Fig. 10b shows a superimposition of the structure and TOPOS representation of part of one (4,4) net in [{Cd(NO3)2(5)2}·2MeOH]n. A comparison of this with Fig. 6a and 7 illustrates that both cis and trans-arrangements of nitrato ligands lead to cavities in the network through which long alkoxy chains from the next sheet can penetrate. This is shown for [{Cd(NO3)2(5)2}·2MeOH]n in Fig. 11a. Just as in [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, 4,2′:6′,4′′-tpy domains in adjacent sheets interact through π-stacking of the central pyridine rings (Fig. 11b). The angle between the least squares planes of the rings containing N2 ad N5i (symmetry code i = 1 − x, 1 − y, 1 − z) is 5.4° and the ring-plane to centroid separation is 3.57 Å. This type of interaction is found in a range of coordination polymers involving 4,2′:6′,4′′-tpy ligands.10Fig. 11b also shows that the depth of penetration of one sheet into the next is such that the 4-phenyl substituent in ligand 5 lies within the (4,4) net of Cd atoms. Again this mimics the situation in the networks involving ligands 3 and 4, and leads to a similar inter-sheet separation; shortest Cd⋯Cd distances between nets are 8.566(2) and 8.950(2) Å with ligand 5versus 8.940(2) and 8.894(2) Å with 3, and 8.468(1) and 8.433(1) Å with 4. Thus, despite the change in the local environment at the Cd centre on going from a trans- to cis-ligand arrangement, the packing interactions between the sheets remain similar.
A change in the ratio of cadmium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) ligand
ligand
For the coordination networks described above, the ratio of cadmium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand in the crystallization experiments was 1
ligand in the crystallization experiments was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 leading to networks with a 2
3 leading to networks with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of Cd
3 ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand 2, or 1
ligand 2, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for ligands 3, 4 or 5. For the hexoxy-tailed ligand 4, a crystallization experiment was also run with a 1
2 for ligands 3, 4 or 5. For the hexoxy-tailed ligand 4, a crystallization experiment was also run with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Cd
1 ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand. Structural analysis (see Fig. S5† for powder diffraction data) revealed the formation of the 1-dimensional coordination polymer [Cd2(NO3)4(MeOH)(4)3]n which crystallizes in the triclinic space group P
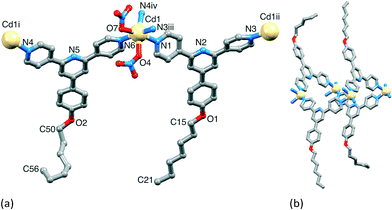
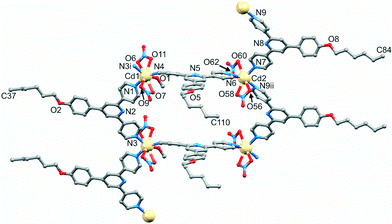
ligand. Structural analysis (see Fig. S5† for powder diffraction data) revealed the formation of the 1-dimensional coordination polymer [Cd2(NO3)4(MeOH)(4)3]n which crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and possesses a ladder structure (Fig. 12 and S6†). The structure is similar to that found for [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n.23 The asymmetric unit contains two independent Cd atoms and three independent ligands 4. Atom Cd1 is 8-coordinate (Fig. 12), binding to three 4,2′:6′,4′′-tpys (Cd1–N1 = 2.316(3), Cd1–N4 = 2.359(4), Cd1–N3i = 2.340(3) Å), an MeOH molecule (Cd1–O1 = 2.428(3) Å) and two bidentate nitrato ligands, although one Cd–O contact is long (Cd1–O6 = 2.754(4), Cd1–O7 = 2.518(4), Cd1–O9 = 2.541(4), Cd1–O11 = 2.421(3) Å). Atom Cd2 is 7-coordinate (Fig. 12), coordinated by three 4,2′:6′,4′′-tpys (Cd2–N9ii = 2.313(4), Cd2–N6 = 2.347(3), Cd2–N7 = 2.302(4) Å) and two bidentate nitrato groups (Cd2–O56 = 2.458(3), Cd2–O58 = 2.455(4), Cd2–O60 = 2.626(4), Cd2–O62 = 2.361(3) Å). The three N-donors at each Cd centre are in a T-shaped arrangement (angles N3i–Cd1–N1 = 104.91(12) and N3i–Cd1–N4 = 93.72(13), N9ii–Cd2–N7 = 103.53(13) and N6–Cd2–N7 = 92.16(13)°).
, and possesses a ladder structure (Fig. 12 and S6†). The structure is similar to that found for [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n.23 The asymmetric unit contains two independent Cd atoms and three independent ligands 4. Atom Cd1 is 8-coordinate (Fig. 12), binding to three 4,2′:6′,4′′-tpys (Cd1–N1 = 2.316(3), Cd1–N4 = 2.359(4), Cd1–N3i = 2.340(3) Å), an MeOH molecule (Cd1–O1 = 2.428(3) Å) and two bidentate nitrato ligands, although one Cd–O contact is long (Cd1–O6 = 2.754(4), Cd1–O7 = 2.518(4), Cd1–O9 = 2.541(4), Cd1–O11 = 2.421(3) Å). Atom Cd2 is 7-coordinate (Fig. 12), coordinated by three 4,2′:6′,4′′-tpys (Cd2–N9ii = 2.313(4), Cd2–N6 = 2.347(3), Cd2–N7 = 2.302(4) Å) and two bidentate nitrato groups (Cd2–O56 = 2.458(3), Cd2–O58 = 2.455(4), Cd2–O60 = 2.626(4), Cd2–O62 = 2.361(3) Å). The three N-donors at each Cd centre are in a T-shaped arrangement (angles N3i–Cd1–N1 = 104.91(12) and N3i–Cd1–N4 = 93.72(13), N9ii–Cd2–N7 = 103.53(13) and N6–Cd2–N7 = 92.16(13)°).
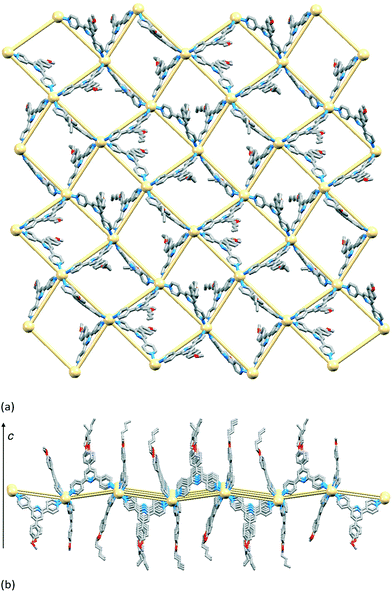
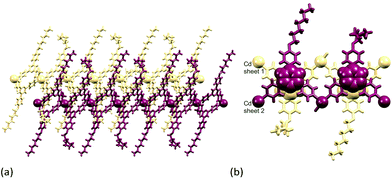
Although topologically the ladders in [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n and [Cd2(NO3)4(MeOH)(4)3]n are the same, the internal angles of each rhombus in the ladder as defined by the ligand-bridged Cd nodes are 82 and 98° in [Cd2(NO3)4(MeOH)(4)3]n (Fig. 13 and S6†), and 27.3 and 152.7° in [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n (Fig. 14). The rails (vertical in Fig. 13 and 14) of the ladder are slipped with respect to one another on going from 4 to 1, and the closest Cd⋯Cd separation across the ladder decreases from 13.0025(8) Å (ligand-bridged) in [Cd2(NO3)4(MeOH)(4)3]n to 6.094(1) Å (unbridged) in [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n.23 The ladder conformation shown in Fig. 14b is also seen in [{Cd2(NO3)4L3}·4MeOH·2CHCl3]n and [{Cd2(NO3)4L3}·2MeOH·3CHCl3·H2O]n in which L = 4′-(4-MeSC6H4)-4,2′:6′,4′′-tpy or 4′-(4-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4)-4,2′:6′,4′′-tpy.24 A comparison of Fig. 13a and 14a reveals that the ligands defining the rails of the ladder in [Cd2(NO3)4(MeOH)(4)3]n (Fig. 13) point outwards from the ladder-framework, in contrast to their orientation in the ladder type found for [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n and its analogues.23,24 Primary packing forces between adjacent coordination polymer chains involve both π-stacking and van der Waals interactions. Adjacent ladders in [Cd2(NO3)4(MeOH)(4)3]n interact through a centrosymmetric face-to-face π-stacking of the central pyridine rings of the ligands 4 forming the rungs of the ladder (Fig. 15); the distance between the N5- and N5iii-containing ring planes (symmetry code iii = −x, 1 − y, 1 − z) is 3.39 Å and the inter-centroid separation is 3.55 Å. The alkoxy chains containing atoms O2 and O8 are in fully extended conformations and chains from adjacent ladders interdigitate with one another as shown in Fig. 15. The terminal methyl group of each 4-hexoxyphenyl unit lies over the arene ring of the next substituent giving a head-to-tail embrace; both independent CMe⋯centroidarene distances are 3.78 Å. Extension of the packing motif in Fig. 15 generates sheets which are locked together by penetration of the protruding, folded hexoxy chains into cavities in the next sheet.
CC6H4)-4,2′:6′,4′′-tpy.24 A comparison of Fig. 13a and 14a reveals that the ligands defining the rails of the ladder in [Cd2(NO3)4(MeOH)(4)3]n (Fig. 13) point outwards from the ladder-framework, in contrast to their orientation in the ladder type found for [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n and its analogues.23,24 Primary packing forces between adjacent coordination polymer chains involve both π-stacking and van der Waals interactions. Adjacent ladders in [Cd2(NO3)4(MeOH)(4)3]n interact through a centrosymmetric face-to-face π-stacking of the central pyridine rings of the ligands 4 forming the rungs of the ladder (Fig. 15); the distance between the N5- and N5iii-containing ring planes (symmetry code iii = −x, 1 − y, 1 − z) is 3.39 Å and the inter-centroid separation is 3.55 Å. The alkoxy chains containing atoms O2 and O8 are in fully extended conformations and chains from adjacent ladders interdigitate with one another as shown in Fig. 15. The terminal methyl group of each 4-hexoxyphenyl unit lies over the arene ring of the next substituent giving a head-to-tail embrace; both independent CMe⋯centroidarene distances are 3.78 Å. Extension of the packing motif in Fig. 15 generates sheets which are locked together by penetration of the protruding, folded hexoxy chains into cavities in the next sheet.
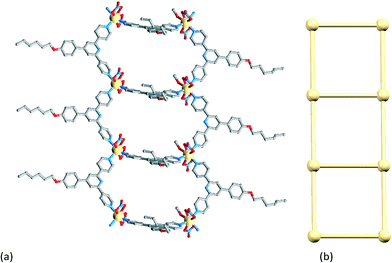 | ||
| Fig. 13 (a) Part of the 1-dimensional coordination polymer in [Cd2(NO3)4(MeOH)(4)3]n and (b) TOPOS representation of part of the ladder. | ||
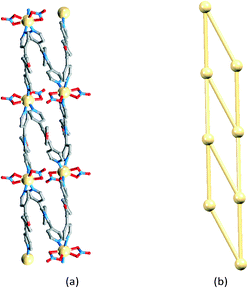 | ||
| Fig. 14 (a) Part of the 1-dimensional coordination polymer in [{Cd2(NO3)4(1)3}·CHCl3·MeOH]n and (b) TOPOS representation of part of a ladder. | ||
 | ||
| Fig. 15 Four ladders in [Cd2(NO3)4(MeOH)(4)3]n viewed down the a-axis showing π-stacking interactions and interdigitation between 4-hexoxyphenyl substituents between adjacent polymer chains. | ||
Solid-state photoluminescence
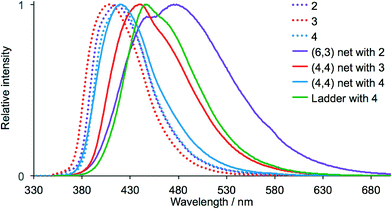
Protonation of 2,2′:6′,2′′-terpyridine (2,2′:6′,2′′-tpy) significantly enhances the photoluminescence (PL) of the ligand in MeCN solution, leading to a red-shift in λemmax from 340 nm to 412 nm, and an increase in quantum yield (QY) from <0.1% to 5.2%.31 Diprotonation results in a further increase in QY.32 It has also been observed that in the solid state, amorphous 2,2′:6′,2′′-tpy and needle-like crystals (melting point 86–88 °C) are non-emissive, while plate-like crystals (melting point 91–93 °C) are photoluminescent (λemmax = 365 nm) with QY = 20% and an emission lifetime of 4.5 ns.33 The emission maxima (Fig. 16) and PL quantum yields of crystalline 2–4 are given in Table 1. All are blue emitters, with QY values ranging from 15 to 27%; the emission lifetimes are <10 ns. Incorporation of the alkoxy-substituent red-shifts the emission with respect to that of solid 4′-phenyl-4,2′:6′,4′′-terpyridine; the latter emits weakly (λemmax = 370 nm) when excited at 345 nm.34 Note that Hou and Li have reported that solid 4′-phenyl-4,2′:6′,4′′-terpyridine exhibits a strong green PL (λemmax = 517 nm, λexc = 400 nm).35| Compound | λ exc/nm | λ maxem/nm | QY/% |
|---|---|---|---|
| 2 | 280 | 416 | 15 |
| 3 | 280 | 408 | 27 |
| 4 | 280 | 420 | 17 |
| [{Cd2(NO3)4(2)3}·3CHCl3]n | 280 | 475 | 11 |
| [{Cd2(NO3)4(3)4}·3CHCl3]n | 280 | 440 | 22 |
| [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n | 280 | 420 | 13 |
| [Cd2(NO3)4(MeOH)(4)3]n | 280 | 447 | 18 |
Fig. 16 shows the emission spectra of solid samples of [{Cd2(NO3)4(2)3}·3CHCl3]n, [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n (all 2-dimensional networks) and of [Cd2(NO3)4(MeOH)(4)3]n (a 1-dimensional ladder). With the exception of [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, the emission broadens and shifts to lower energy on going from free ligand to complex, but remains in the blue region. Similar red-shifts have been reported when N-donor ligands coordinate to zinc(II) or cadmium(II), and the emissions are assigned to intra-ligand transitions.34,36 On going from 4 to [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n, the emission band broadens but λmaxem remains at 420 nm. The formation of the coordination polymers incorporating 2, 3 or 4 has little effect on the QYs compared to those of the free ligands, and for each complex, the emission lifetime is <10 ns.
Conclusions
The divergent N,N-binding mode of 4,2′:6′,4′′-terpyridines is readily exploited for the formation of coordination polymers and networks, but determining factors that can encourage the formation of 2- and 3-dimensional networks remains under-developed. In this work, we have demonstrated the structural consequences of increasing the length of the alkoxy substituent in 4′-alkoxy-4,2′:6′,4′′-terpyridines when these ligands combine with Cd(NO3)2·4H2O with a Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 1
ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Ligand 2 contains a 4′-n-propoxy substituent and forms [{Cd2(NO3)4(2)3}·3CHCl3]n consisting of a (6,3) net. n-Propoxy chains protrude from the sheet and are involved in ‘tail-in-pocket’ interactions which interlock the sheets. Although preliminary data indicate that an n-butoxy chain causes no major structural perturbation, introduction of longer chains cause a switch from a (6,3) to (4,4) net. The change is consistent with the pockets in which the smaller chains are accommodated when (6,3) sheets pack together are too small to accommodate longer chains. In [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n each 4-connecting Cd atom has a trans-arrangement of nitrato ligands, while in [{Cd(NO3)2(5)2}·2MeOH]n, they are cis. The reaction between Cd(NO3)2·4H2O and 4 using a 1
3. Ligand 2 contains a 4′-n-propoxy substituent and forms [{Cd2(NO3)4(2)3}·3CHCl3]n consisting of a (6,3) net. n-Propoxy chains protrude from the sheet and are involved in ‘tail-in-pocket’ interactions which interlock the sheets. Although preliminary data indicate that an n-butoxy chain causes no major structural perturbation, introduction of longer chains cause a switch from a (6,3) to (4,4) net. The change is consistent with the pockets in which the smaller chains are accommodated when (6,3) sheets pack together are too small to accommodate longer chains. In [{Cd2(NO3)4(3)4}·3CHCl3]n and [{Cd2(NO3)4(4)4}·CHCl3·MeOH]n each 4-connecting Cd atom has a trans-arrangement of nitrato ligands, while in [{Cd(NO3)2(5)2}·2MeOH]n, they are cis. The reaction between Cd(NO3)2·4H2O and 4 using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Cd
1 ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand switches the assembly to a 1-dimensional ladder; in [Cd2(NO3)4(MeOH)(4)3]n each Cd atom is a 3-connecting node. Face-to-face π-interactions between arene rings (either pyridine/pyridine or pyridine/phenyl) in adjacent sheets or chains are a common feature of the coordination networks, and van der Waals interactions between n-hexoxy chains play a dominant role in the packing of ladders in [Cd2(NO3)4(MeOH)(4)3]n. In the solid state, the coordination polymers are blue emitters; values of λmaxem are red-shifted up to 59 nm with respect to the free ligand, and quantum yields are in the range 11–22%.
ligand switches the assembly to a 1-dimensional ladder; in [Cd2(NO3)4(MeOH)(4)3]n each Cd atom is a 3-connecting node. Face-to-face π-interactions between arene rings (either pyridine/pyridine or pyridine/phenyl) in adjacent sheets or chains are a common feature of the coordination networks, and van der Waals interactions between n-hexoxy chains play a dominant role in the packing of ladders in [Cd2(NO3)4(MeOH)(4)3]n. In the solid state, the coordination polymers are blue emitters; values of λmaxem are red-shifted up to 59 nm with respect to the free ligand, and quantum yields are in the range 11–22%.
Acknowledgements
We thank the Swiss National Science Foundation, the European Research Council (Advanced Grant 267816 LiLo) and the University of Basel for financial support. The Swiss National Science Foundation through the NCCR Molecular Systems Engineering is acknowledged for partial funding of the powder diffractometer. Dr Collin D. Morris is thanked for assistance with powder diffraction and photoluminescence measurements.Notes and references
- P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS.
- S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS PubMed.
- S. R. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972 CrossRef CAS PubMed.
- B. H. Northrop, H. B. Yang and P. J. Stang, Chem. Commun., 2008, 5896 RSC.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418 CrossRef CAS PubMed.
- E. C. Constable in Supramolecular Chemistry: From Molecules to Nanomaterials, ed. P. A. Gale and J. W. Steed, Wiley, 2012, vol. 6, p. 3073 Search PubMed.
- M. O'Keefe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed.
- E. C. Constable, Pure Appl. Chem., 1996, 68, 253 CrossRef CAS.
- E. C. Constable, Chem. Soc. Rev., 2007, 36, 246 RSC.
- C. E. Housecroft, Dalton Trans., 2014, 43, 6594 RSC.
- X. Tan, X. Chen, J. Zhang and C.-Y. Song, Dalton Trans., 2012, 41, 3616 RSC; L. Wen, X. Ke, L. Qiu, Y. Zou, L. Zhou, J. Zhao and D. Li, Cryst. Growth Des., 2012, 12, 4083 Search PubMed; W. Yang, A. J. Davies, X. Lin, M. Suyetin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, J. E. Parker, C. C. Tang, M. W. George, P. Hubberstey, S. Kitagawa, H. Sakamoto, E. Bichoutskaia, N. R. Champness, S. Yang and M. Schröder, Chem. Sci., 2012, 3, 2992 Search PubMed; Y.-Q. Chen, S.-J. Liu, Y.-W. Li, G.-R. Li, K.-H. He, Y.-K. Qu, T.-L. Hu and X.-H. Bu, Cryst. Growth Des., 2012, 12, 5426 Search PubMed; P. Yang, M.-S. Wang, J.-J. Shen, M.-X. Li, Z.-X. Wang, M. Shao and X. He, Dalton Trans., 2014, 43, 1460 RSC; Y.-L. Gai, F.-L. Jiang, L. Chen, Y. Bu, M.-Y. Wu, K. Zhou, J. Pan and M.-C. Hong, Dalton Trans., 2013, 42, 9954 RSC; Y.-Q. Chen, G.-R. Li, Y.-K. Qu, Y.-H. Zhang, K.-H. He, Q. Gao and X.-H. Bu, Cryst. Growth Des., 2013, 13, 901 Search PubMed; Y. Li, Z. Ju, B. Wu and D. Yuan, Cryst. Growth Des., 2013, 13, 4125 Search PubMed; F. Yuan, J. Xie, H.-M. Hu, C.-M. Yuan, B. Xu, M.-L. Yang, F.-X. Dong and G.-L. Xue, CrystEngComm, 2013, 15, 1460 RSC; F. Yuan, Q. Zhu, H.-M. Hu, J. Xie, B. Xu, C.-M. Yuan, M.-L. Yang, F.-X. Dong and G.-L. Xue, Inorg. Chim. Acta, 2013, 397, 117 CrossRef CAS PubMed; H.-N. Zhang, F. Yuan, H.-M. Hu, S.-S. Shen and G.-L. Xue, Inorg. Chem. Commun., 2013, 34, 51 CrossRef PubMed; E. C. Constable, G. Zhang, C. E. Housecroft and J. A. Zampese, CrystEngComm, 2011, 13, 6864 RSC; J. Heine, J. Schmedt auf der Günne and S. Dehnen, J. Am. Chem. Soc., 2011, 133, 10018 CrossRef PubMed; V. N. Dorofeeva, S. V. Kolotilov, M. A. Kiskin, R. A. Polunin, Z. V. Dobrokhotova, O. Cador, S. Golhen, L. Ouahab, I. L. Eremenko and V. M. Novotortsev, Chem. – Eur. J., 2012, 18, 5006 CrossRef PubMed; K.-R. Ma, F. Ma, Y.-L. Zhu, L.-J. Yu, X.-M. Zhao, Y. Yang and W.-H. Duan, Dalton Trans., 2011, 40, 9774 RSC.
- E. C. Constable, C. E. Housecroft, P. Kopecky, M. Neuburger, J. A. Zampese and G. Zhang, CrystEngComm, 2012, 14, 446 RSC.
- E. C. Constable, G. Zhang, C. E. Housecroft and J. A. Zampese, Inorg. Chem. Commun., 2012, 15, 113 CrossRef CAS PubMed.
- Y. M. Klein, E. C. Constable, C. E. Housecroft, J. A. Zampese and A. Crochet, CrystEngComm, 2014, 16, 9915 RSC.
- E. C. Constable, C. E. Housecroft, S. Vujovic and J. A. Zampese, CrystEngComm, 2014, 16, 3494 RSC.
- Y. M. Klein, E. C. Constable, C. E. Housecroft and A. Prescimone, CrystEngComm, 2015, 17, 2070 RSC.
- E. C. Constable, C. E. Housecroft, M. Neuburger, J. Schönle, S. Vujovic and J. A. Zampese, Polyhedron, 2013, 60, 120 CrossRef CAS PubMed.
- E. C. Constable, G. Zhang, E. Coronado, C. E. Housecroft and M. Neuburger, CrystEngComm, 2010, 12, 2139 RSC.
- E. C. Constable, C. E. Housecroft, S. Vujovic, J. A. Zampese, A. Crochet and S. R. Batten, CrystEngComm, 2013, 15, 10068 RSC.
- V. Auger and I. Robin, Comptes Rendus, 1924, 178, 1546 CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS PubMed.
- E. C. Constable, G. Zhang, C. E. Housecroft, M. Neuburger and J. A. Zampese, CrystEngComm, 2009, 11, 2279 RSC.
- Y. M. Klein, E. C. Constable, C. E. Housecroft and A. Prescimone, Inorg. Chem. Commun., 2014, 49, 41 CrossRef PubMed.
- E. C. Constable, G. Zhang, C. E. Housecroft, M. Neuburger and J. A. Zampese, CrystEngComm, 2010, 12, 3733 RSC.
- APEX2, version 2 User Manual, M86-E01078, Bruker Analytical X-ray Systems, Inc., Madison, WI, 2006 Search PubMed.
- P. W. Betteridge, J. R. Carruthers, R. I. Cooper, K. Prout and D. J. Watkin, J. Appl. Crystallogr., 2003, 36, 1487 CrossRef CAS.
- I. J. Bruno, J. C. Cole, P. R. Edgington, M. K. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389 CrossRef PubMed.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466 CrossRef CAS.
- V. A. Blatov and A. P. Shevchenko, TOPOS Professional v. 4.0, Samara State University, Russia Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- N. Yoshikawa, S. Yamabe, N. Kanehisa, H. Takashima and K. Tsukahara, J. Phys. Org. Chem., 2009, 22, 410 CrossRef CAS PubMed.
- N. Yoshikawa, S. Yamabe, N. Kanehisa, T. Inoue, H. Takashima and K. Tsukahara, J. Phys. Org. Chem., 2010, 23, 431 CAS.
- T. Mutai, H. Satou and K. Araki, Nat. Mater., 2005, 4, 685 CrossRef CAS PubMed.
- E. C. Constable, G. Zhang, C. E. Housecroft, M. Neuburger and J. A. Zampese, CrystEngComm, 2010, 12, 2146 RSC.
- L. Hou and D. Li, Inorg. Chem. Commun., 2005, 8, 190 CrossRef CAS PubMed.
- See for example: W. Li, H.-P. Jia, Z.-F. Ju and J. Zhang, Cryst. Growth Des., 2006, 6, 2136 Search PubMed; G.-X. Jin, J.-P. Ma and Y.-B. Dong, J. Mol. Struct., 2013, 1052, 146 CrossRef CAS PubMed; C.-M. Che, C.-W. Wan, K.-Y. Ho and Z.-Y. Zhou, New J. Chem., 2001, 25, 63 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S5: powder diffraction data; Fig. S6: part of the coordination polymer chain in [Cd2(NO3)4(MeOH)(4)3]n. CCDC 1402482–1402486. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2dt12242a |
| This journal is © The Royal Society of Chemistry 2015 |