Reactive organogels based on isoxazole esters: alkali metal ions selective gelation and crystallization†
Abstract
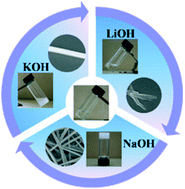
A series of simple ester molecules containing an isoxazole moiety were found to form instant organogels at room temperature in the presence of NaOH without the heating-cooling cycle used for conventional supramolecular gels. The gelation process was triggered due to the hydrolysis of the isoxazole esters and occurred selectively with Na+. When LiOH, NaOH and KOH were separately introduced into the methanol solutions of the isoxazole esters, the solution remained as a solution, transformed to a organogel and a crystal, respectively. With the help of a study on the phase behavior of the corresponding isoxazole acid in the presence of the alkali bases, it was revealed that π–π stacking of the isoxazole moiety and the ionic interaction between the carboxylates and Na+ are the main driving forces for the self-assembly and the organogelation. The size of the alkali metal ions will subtly affect the gelation, with the Li+ and K+ ions leading to solution and crystallization, respectively. These results have provided an insight into the balance between the solution, gelation and crystallization with subtle molecular variations.
- This article is part of the themed collection: Supramolecular Gels in Crystal Engineering

 Please wait while we load your content...
Please wait while we load your content...